AbstractTherapeutic hypothermia (TH) has rarely been utilized as an adjunct to anticonvulsants in treating patients with refractory convulsive status epilepticus (CSE). However, determining the effectiveness of TH in CSE is difficult due to the unavoidable use of sedative drugs to manage hypothermia. Additionally, the effectiveness of TH has not been studied in patients with refractory non-convulsive status epilepticus (NCSE). Here, we report the successful use of TH without additional sedative drugs in a patient with temporal lobe epilepsy and refractory NCSE. A 46-year-old man was referred to the neurology department because of recurrent seizure attacks. Electroencephalography (EEG) after first-line status treatment showed continuous periodic discharges consistent with NCSE. He was started simultaneously on continuous EEG monitoring and TH, but was not administered any benzodiazepines to control shivering or maintain TH. During TH, EEG abnormalities gradually improved, and the patient regained consciousness in accordance with the improvement in EEG. The patient was alert and his EEG had normalized a few days after starting TH. To the best of our knowledge, this is the first report describing the successful treatment of refractory NCSE with TH. As no sedative drugs were used during the maintenance of hypothermia, NCSE control may have been achieved by TH alone.
IntroductionNonconvulsive status epilepticus (NCSE) is defined as a change in mental state from baseline, lasting at least 30–60 minutes, associated with continuous or near-continuous epileptiform discharge on the electroencephalogram (EEG).1 NCSE is thought to constitute 20–25% of all episodes of status epilepticus (SE).2 Refractory SE (RSE) has been associated with high morbidity and mortality rates, and does not always respond to standard pharmacological treatments. When these treatments fail, more active and aggressive alternative treatments are required.
Systemic therapeutic hypothermia (TH) has shown benefits in various clinical settings, including post-cardiac arrest and neonatal hypoxic encephalopathy, and has been found to control intracranial pressure in patients with traumatic brain injury and stroke.3–5 Experimentally, TH was shown to have anticonvulsant and neuroprotective effects after SE.6–7 Although several case reports have shown promising results following the application of TH in patients with RSE,8–10 TH was used as adjuvant therapy in almost all these patients. This report describes the successful use of TH without additional sedative drugs in a patient with temporal lobe epilepsy who experienced refractory NCSE. Findings in this patient suggest that TH can be considered an adjunctive or alternative therapy in patients with medically intractable NCSE.
CaseA 46-year-old man was referred to the neurology department of Samsung Changwon Hospital because of recurrent generalized tonic-clonic (GTC) seizures. At that time, the patient was undergoing wound care at the Orthopedics Department of our hospital because of necrotizing fasciitis of the right foot. GTC seizures were observed twice by station nurses. The duration of each seizure was slightly over 5 minutes, with the patient not recovering mental status between seizures. After the second GTC seizure, the patient was administered a loading dose of lorazepam, but he remained unconscious, and remnant subtle motor components were observed. The patient’s care-giver informed us that the patient had a previous history of chronic alcoholism and was later diagnosed with diabetes as a post-alcoholic complication of pancreatitis. He had a history of alcohol-related seizures since his late 20s and was diagnosed in his early 30s with temporal lobe epilepsy accompanied by left hippocampal sclerosis. Despite treatment with valproate 600 mg bid, levetiracetam 1,000 mg bid, and vigabatrin 500 mg bid, he was not seizure-free. He was administered 1 mg/kg intravenous lorazepam, followed by a loading dose of 15 mg/kg intravenous fosphenytoin (15 mg/kg). He continued to receive his usual doses of valproate, levetiracetam, and vigabatrin, administered through a nasogastric feeding tube. After lorazepam loading, however, his valproate dose was changed to 450 mg intravenous every 8 hours, and levetiracetam dose to 1,500 mg bid, with no change in vigabatrin dose.
Although his convulsive seizures ceased, the patient did not recover consciousness. An initial EEG showed continuous periodic discharges in both posterior cerebral hemispheres (Fig. 1A). Diffusion-weighted magnetic resonance imaging (MRI) (Fig. 2A, B) showed symmetric diffusion-restricted lesions in the bilateral thalamus and parieto-occipital cortex with low-signal changes in apparent diffusion coefficient images (Fig. 2C, D). Several follow-up EEGs showed continuous, periodic single or poly-spikes and waves in the occipital areas (Fig. 1B). He was therefore administered loading doses of 600 mg pregabalin and 15 mg/kg phenobarbital on days of hospital stay 2 and 5, respectively (Fig. 3).
On day of hospital stay 6, the patient was started on continuous EEG monitoring and TH, without any additional medication to control seizures. At the time of starting TH, the patient was in a semi-comatose and intubated state. TH at a target temperature of 35°C was administered using an Arctic Sun 5000® temperature management system (Medivance Arctic Sun System, Louisville, CO, USA), which uses external gel pads and circulating sterile water. The induction phase lasted 8 hours and the maintenance phase lasted 24 hours at 35°C. The patient was monitored with esophageal core probes, and normothermia was achieved by controlled rewarming over 8 hours. The patient was not administered any benzodiazepine drugs to control shivering or to maintain hypothermia. Rather, the patient was maintained by intravenous infusion of remifentanil hydrochloride, 2,600 mg/day acetaminophen and 11 mg/day buspirone.
EEG after induction of hypothermia to 35°C showed that the periodic epileptiform discharges observed in this patient had decrease in amplitude without a definite change in pattern (Fig. 1C). During maintenance of hypothermia, EEG abnormalities gradually improved and the patient regained consciousness in proportion to the improvement in EEG (Fig. 1D, E). The patient was alert and his EEG normalized on the second day following initiation of the rewarming procedure. Follow-up MRI two weeks after TH showed normalization of the areas with initially-reduced diffusion (Fig. 4).
This case report was approved by the institutional review boards (IRB No. 2016-07-032) of the Sungkyunkwan University Samsung Changwon Hospital. The patient and his caregivers provided consent for publication of this report.
DiscussionAfter controlling generalized convulsive SE in our patient, he was successfully treated with TH for refractory NCSE. The absence of additional sedative drugs, except for remifentanil hydrochloride, during hypothermia maintenance indicates that TH itself may have controlled his refractory NCSE. Although TH has been used as adjunct therapy for patients with refractory epilepsy and SE,8,11,12 patients with refractory convulsive SE may require sedative drugs to control shivering and maintain hypothermia. Simultaneous administration of sedative drugs limits the ability to evaluate the effects of hypothermia alone. Because our patient did not experience convulsions, he was not treated with any additional drugs that could have contributed to improvements in EEG or clinical symptoms. Findings from our patient suggest that mild TH is an appropriate treatment for refractory NCSE and should be considered before administering general anesthetics to control seizures.
The use of TH has increased worldwide, as this technique was found to be advantageous in a variety of clinical settings. Examples include unconscious adult patients with spontaneous circulation after an out-of-hospital cardiac arrest, near-drowning, traumatic head injury, hepatic encephalopathy, and stroke.3–5 Experimental studies showed that TH has anti-epileptic and neuroprotective effects, leading to its clinical use to treat SE.6–7 The neurophysiological processes involved in the anticonvulsant effects of hypothermia are still largely unclear. However, there are theoretical reasons to recommend hypothermia to treat SE. Hypothermia was first reported successful in treating three children with SE under thiopental anesthesia.8 Case series and case reports have shown that TH is safe and effective in patients with RSE.8,13–15 Complications of TH include dysrhythmias, infections, and primary coagulopathy, but these complications were reported in patients subjected to severe hypothermia (< 32°C), or when there were problems controlling temperature.3,4 Because mild TH has no significant adverse effects, it is regarded as relatively safe and easy to use.16 As our patient was thought to be less responsive to conventional AEDs, mild TH was deemed a possible option for managing NCSE prior to applying general anesthesia.
NCSE is defined as a state of ongoing seizures without convulsions, usually for longer than 30 minutes, associated with continuous epileptiform discharges on EEG.1 NCSE constitutes 20% to 25% of episodes of SE17,18 and occurs in approximately 8% of all comatose patients without clinical signs of seizure activity.19 NCSE has been found to persist in 14% of patients after generalized convulsive SE is controlled.20 Although there is no generally accepted clinical definition of NCSE, early diagnosis is required to prevent potential neurological damage. Diagnosis of NCSE requires a combination of clinical and EEG features and particularly relies on EEG findings.21 Various EEG patterns have been described in NCSE, some of which clearly denote NCSE. In contrast, other patterns, such as triphasic waves, generalized periodic epileptiform discharges, and periodic lateralized epileptiform discharges (PLEDs), may not be indicative of NCSE.22 NCSE has been diagnosed in patients with PLEDs who meet certain criteria that occur in comatose patients in the aftermath of generalized tonic-clonic SE.21 Our patient was diagnosed with NCSE based on these criteria. Additionally, because the EEG and mental status of our patient gradually improved after hypothermia treatment, this improvement may also be diagnostic of NCSE.
Some NCSE case series have reported high mortality and morbidity rates,23–25 suggesting that aggressive therapy is warranted. A study of 100 patients with NCSE found that 18 had died,26 with mortality associated with acute medical causes, impairment of mental status, and development of acute complications.24 However, there is no consensus on robust treatments for NCSE, unlike SE. Therefore, treatment of NCSE depends on the type and cause.27 Because some patients with NCSE have shown more favorable outcomes than patients with convulsive SE, caution should be used prior to further treatment with intravenous anesthetics.28,29 Aggressive pharmacological treatment appears to have a greater risk of morbidity and mortality than continuing non-convulsive seizure activity.30 Additionally, non-pharmacological treatments, such as hypothermia, vagal nerve stimulation, and electroconvulsive treatment, are recommended for patients with NCSE, although evidence is insufficient. Therefore, patients, such as ours, treated with various antiepileptic drugs with loading doses for a sufficient time to achieve the maximum effect, and who show no improvement in mental status or EEG may require intravenous anesthetics or other non-pharmacological treatments.
Because SE has heterogeneous causes, which greatly determine prognosis, the response of SE to treatment is difficult to evaluate using consistent and uniform criteria. However, studies have reported that TH has seizure cessation and reduction rates of 62.5% and 15%, respectively, indicating that rate of seizure response in patients with RSE is relatively high (77.5%).10 No correlation has been observed between target temperature and seizure response, indicating the need for additional studies on the effects, suitable target temperatures, and complications of TH. Furthermore, due to the development of TH tools with few serious adverse effects, the use of mild TH in patients with refractory NCSE should be considered in lieu of intravenous anesthetics.
Conflicts of InterestConflicts of interest The authors have no relevant conflict of interest to report. Figure 1Ten-second EEG trace recordings with a 10–20 electrode system (A) following initial treatment of convulsive status epilepticus with lorazepam and fosphenytoin loading and showing continuous quasi-periodic single or poly-spikes and waves of high amplitude, mainly in both occipitoparietal areas; (B) after phenobarbital loading and just before induction of TH; (C) following induction of hypothermia to the 35°C target temperature; and after (D) 12 hours and (E) 24 hours of hypothermia at 35°C. EEG, electroencephalography. 
Figure 2Brain (A, B) diffusion MRI and (C, D) ADC maps after initial treatment for convulsive status epilepticus. Both showed symmetric restricted diffusion in bilateral occipitoparietal areas. MRI, magnetic resonance imaging; ADC, apparent diffusion coefficient. 
Figure 3Summary of the clinical condition of our patient, including EEG, body temperature, and history of treatment with antiepileptic drugs. CSE, convulsive status epilepticus; NCSE, non-convulsive status epilepticus; EEG, electroencephalography; PHT, phenytoin; LEV, levetiracetam; VPA, valproic acid; VGB, vigabatrin; SE, status epilepticus. 
Figure 4(A) Brain diffusion, (B) ADC, (C) fluid-attenuated inversion recovery (FLAIR), and (D) T1 enhanced MRI after 2 weeks of TH. Follow-up diffusion MRI and the ADC map showed normalization of the areas with initially reduced diffusion. FLAIR and T1 enhanced MRI showed no abnormal findings. ADC, apparent diffusion coefficient; FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance imaging; TH, therapeutic hypothermia. 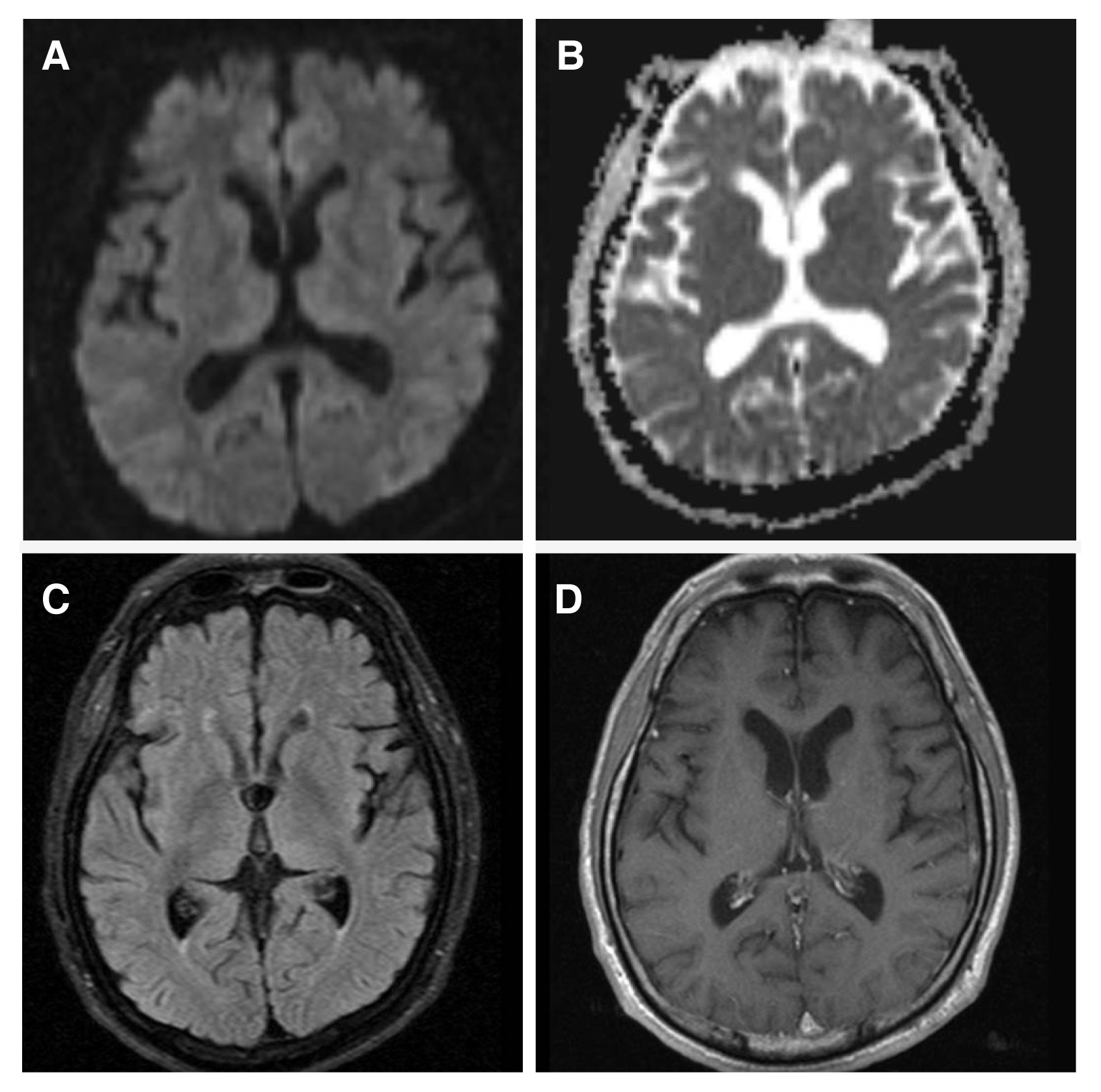
References2. Alroughani R, Javidan M, Qasem A, Alotaibi N. Non-convulsive status epilepticus; the rate of occurrence in general hospital. Seizure. 2009;18:38–42.
3. Nolan JP, Hazinski MF, Steen PA, Becker LB. Controversial topics from the 2005 International Consensus Conference on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2005;67:175–9.
4. Safar PJ, Kochanek PM. Therapeutic hypothermic after cardiac arrest. N Engl J Med. 2002;346:612–3.
6. Schmitt FC, Buchheim K, Meierkord H, Holtkamp M. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis. 2006;23:689–96.
7. Yu L, Zhou Y, Chen W, Wang Y. Mild hypothermia pretreatment protects against pilocarpine-induced status epilepticus and neuro-nalapoptosis in immature rats. Neuropathology. 2011;31:144–51.
8. Orlowski JP, Erenberg G, Lueders H, Cruse RP. Hypothermia and barbiturate coma for refractory status epilepticus. Crit Care Med. 1984;12:367–72.
9. Corry JJ, Dhar R, Murphy T, Diringer MN. Hypothermia for refractory status epilepticus. Neurocrit Care. 2008;9:189–97.
10. Zeiler FA, Zeiler KJ, Teitelbaum J, Gillman LM, West M. Therapeutic hypothermia for refractory status epilepticus. Can J Neurol Sci. 2015;42:221–9.
11. Bennett AE, Hoesch RE, DeWitt LD, Afra P, Ansari SA. Therapeutic hypothermia for status: a report, historical perspective, and review. Clin Neuro Neurosurg. 2014;126:103–9.
12. Zhumadilov A, Gilman CP, Viderman D. Management of super-refractory status epilepticus with isoflurane and hypothermia. Front Neurol. 2015;5:286
13. Bagić A, Theodore WH, Boudreau EA, et al. Towards a non-invasive interictal application of hypothermia for treating seizures: a feasibility and pilot study. Acta Neurol Scand. 2008;118:240–4.
14. Cereda C, Berger MM, Rossetti AO. Bowel ischemia: a rare complication of thiopental treatment for status epilepticus. Neurocrit Care. 2009;10:355–8.
15. Harbert MJ, Tam EW, Glass HC, et al. Hypothermia is correlated with seizure absence in perinatal stroke. J Child Neurol. 2011;26:1126–30.
16. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56.
18. Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia. 2010;51:251–6.
19. Towne AR, Waterhouse EJ, Boggs JN, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–5.
20. DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after control of convulsive status epilepticus. Epilepsia. 1998;38:833–40.
21. Sutter R, Kaplan WP. Electroencephalographic criteria for non-convulsive status epilepticus: synopsis and comprehensive survey. Epilepsia. 2012;53: Suppl 3. 1–51.
22. Yu X, Shao X, Sun H, Zhong C, Cai J, Gao L. Nonconvulsive status epilepticus associated with periodic lateralized epileptiform discharges in a patient with syphilis. Interdiscip Neurosurg. 2016;5:35–7.
23. Treiman DM, Delgado-Escueta AV, Clark MA. Impairment of memory following prolonged complex partial status epilepticus. Neurology. 1981;31:109
24. Krumholz A, Sung GY, Fisher RS, Barry E, Bergey GK, Grattan LM. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology. 1995;45:1499–504.
25. Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83–9.
26. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 2003;61:1066–73.
27. Meierkord H, Holtkamp M. Non-convulsive status epilepticus in adults: clinical forms and treatment. Lancet Neurol. 2007;6:329–39.
28. Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav. 2000;1:301–14.
|
|
|||||||||||||||||||||||||||||||||||||||