Introduction
Over 50% of the world’s population has been reported to be bilingual (i.e., theoretically presenting equal fluency in two languages).1 The epilepsy affects 1% of the population by age 20 and 3% of the population by age 75.2 Consequently, the epilepsy patients are not uncommon in bilingual population. Among the patients with epilepsy, temporal lobe epilepsy (TLE) is the most common symptomatic partial epilepsy in adolescents and adults. It represents almost two thirds of cases of intractable epilepsy managed surgically. A history of febrile seizures (especially complex febrile seizures) is common in TLE and is frequently associated with mesial temporal sclerosis (the commonest form of TLE).3
Speech disturbances are observed in the majority of temporal lobe complex motor seizures. Types of language disturbances include vocalizations, abnormal speech, ictal speech, and postictal aphasia.4 With verbal automatism, increased verbal outputs are often linguistically correct, reiterative, and occur spontaneously.5 It is frequently associated with seizures originating in the temporal lobe, arising from the language non-dominant side.6 Seizure auras occur in many TLE patients and often exhibit features that are relatively specific for TLE but few are of lateralizing value. Automatisms, however, often have lateralizing significance. Careful study of seizure semiology remains invaluable in addressing the search for the seizure onset zone.3 Our case report of foreign language ictal speech automatisms is a rare ictal sign in temporal lobe epilepsy arising from the non-dominant hemisphere.
Case
A 45-year-old (right-handed) Korean woman presented an abrupt motionless stare looking, altering her ability to interact with her environment and speak of foreign language. These episodes lasted a few minutes and were preceded by aura (feeling of dream and floating of the body). Furthermore, she did not remember these events during the postictal period. She has been diagnosed as complex partial seizures since 2nd grade of middle school age. Her seizures came out 2 to 3 times per month on combination of oxcarbazepine and levetiracetam and which were precipitated by stress. She had the history of febrile convulsion on early childhood. She majored in foreign language (Chinese) but she speaks her mother tongue (Korean) exclusively. There was no family history of epilepsy and head injury.
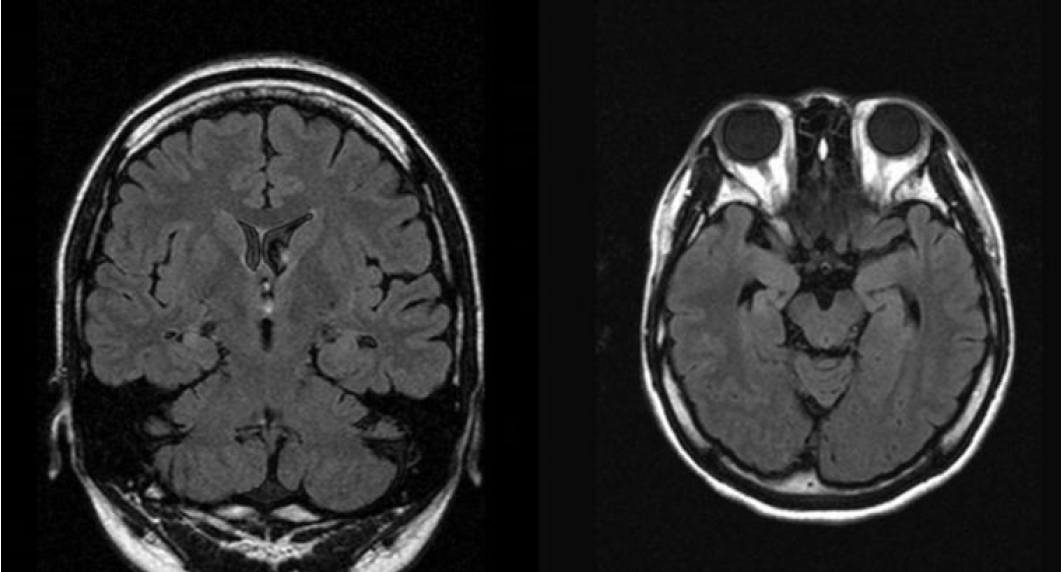
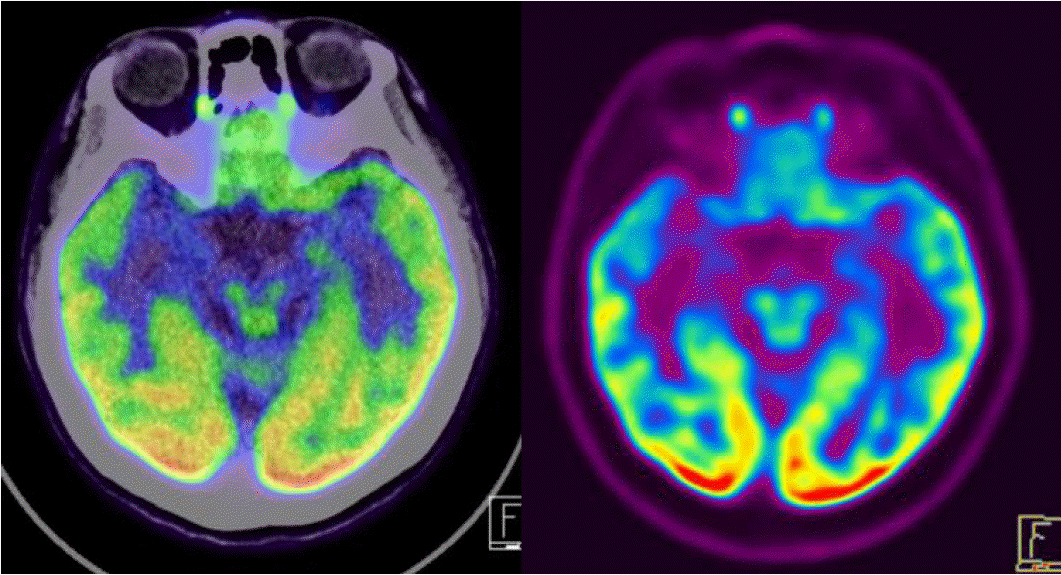
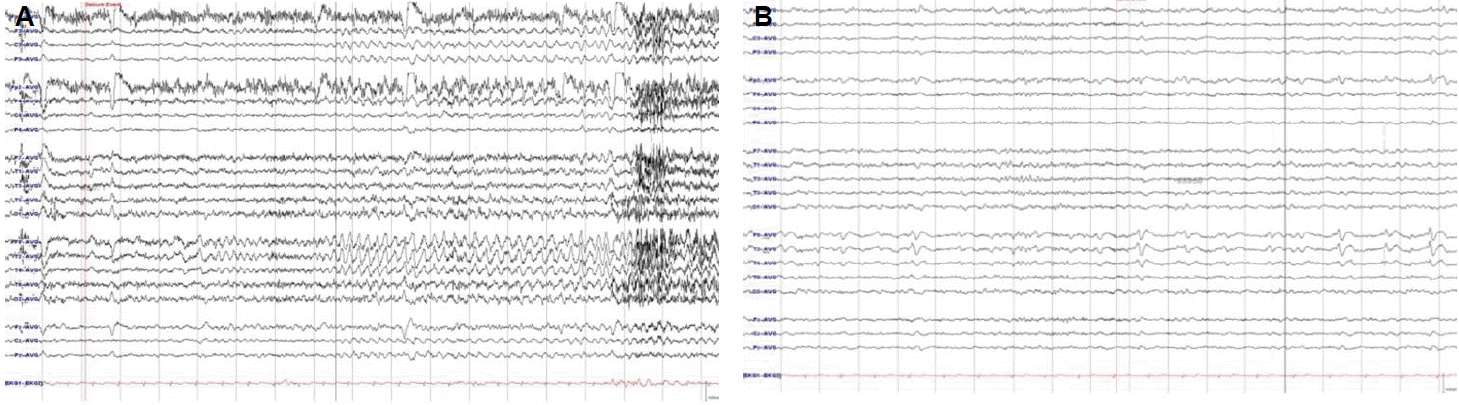
On examination, the patient was alert and oriented. There were no abnormalities on neurological examination. Brain magnetic resonance imaging (MRI) revealed the right hippocampus sclerosis of mild degree (Fig. 1). Her brain FDG Fusion PET scan showed relative hypometabolism in the right medial temporal cortex (Fig. 2). On video EEG monitoring, she spoke simple comprehensive Chinese language unconsciously. Her ictal EEG showedrhythmic theta activities evolving into repetitive sharp waves at the right temporal area (Fig. 3A) which coincided with Chinese language speech automatisms. Additionally, her interictal EEG showed spike-wave complex at right temporal region (Fig. 3B).
Discussion
The symptomatology of auras and seizures is usually a reflection of activation of specific parts of the brain by the ictal discharge, the location and extent of which represent the symptomatogenic zone.4
Ictal speech, defined as clearly intelligible speech during the period of altered consciousness, was localized to the non-dominant temporal lobe.5 Ictal verbalization had significant lateralization value: 90% of patients with this manifestation had seizure focus in the non-dominant temporal lobe.7 Ictal speech automatisms are usually uttered in the patient’s native language, but rare cases of second language (L2) ictal speech production have been reported. Native language and second language ictal speech automatisms generally result from partial seizures that originate in the non-dominant hemisphere.8–10
Our patient presented with foreign language ictal speech automatisms is a rare ictal manifestation of epilepsy. Foreign language ictal speech automatisms are more likely to occur in men with nondominant amygdala onset seizures, an observation that might reflect the sexual dimorphism observed in the right amygdala during emotional processing.11 These findings were different from sex distribution with our report. However, one of the case report found theforeign language ictal speech automatisms in teenage girl.12
The distinct brain regions could be specifically involved in different languages was born.13 There are differences in brain activation depending on the language proficiency and on the age of language acquisition. In highly proficient bilingual individuals with early acquisition of L2, both languages activate essentially identical cortical networks,14 and the selection and control of the output language are thought to involve subcortical structures, notably the left caudate.15 In contrast, for subjects with low proficiency or late acquisition (after the age of 6) of L2, cortical regions activated by L2 are more extended,16 with an occasional right hemispheric predominance.17 In one study, the authors found both language-specific sites and language-common sites but the language-specific sites were exclusively located in temporo-parietal areas.18
In our case, we found that ictalonset of foreign language speech automatisms was arising from the non-dominant temporal lobe during video EEG recording. This finding was the same with previous case reports.12,19,20
There were two possible explanations of this lateralization pattern: either ictal speech in L2 results from release of the dominant hemisphere from the inhibitory action of the non-dominant one, or ictal speech results directly from overactivation of the non-dominant hemisphere.9 According to these hypotheses, the brain systems involved in L2 ictal speech, were delineated by combined functional MRI during bilingual tasks and ictal - interictal single-photon emission computed tomography in a patient who presented L2 ictal speech productions. These analyses showed that the networks activated by the seizure and those activated by L2 processing intersected in the right hippocampus.19 In our case, we found that relative hypometabolism in the right medial temporal cortex in brain FDG Fusion PET scan.
However, in the consideration of language as a highly organized function, it seems not possible that the direct activation of language area on the right hemisphere can generate L2 speech automatism. The fact that direct cortical stimulation inhibits language generation also supports the infeasibility of speech generation by ictal activation. If the direct activation of language cortex is the key mechanism of ictal speech automatism, this semiology should be observed much more commonly in the left temporal lobe epilepsy.
The existence of distinct cerebral areas for different languages in bilinguals has been supported by neuroimaging, direct electrical stimulation, or lesion studies especially in late bilinguals.18,21–24 The release of isolated L2 language area could be possible depending on the pattern of ictal spreading of non-dominant hemisphere if the reciprocal inhibition of bilateral hemispheres is present by point-to-point manners.