AbstractBackground and Purpose:Heart rate (HR) change is easily seen in seizures. Tachycardia is frequently seen in temporal lobe epilepsy (TLE), rather than extra temporal lobe epilepsy (XTLE). We report the difference in the HR pattern between TLE and frontal lobe epilepsy (FLE) during peri-ictal period.
Methods:The ECG data, collected during EEG monitoring, was used. To compare the HR pattern between FLE and TLE, we investigated the baseline HR, maximum HR, seizure onset to peak HR, HR change, and the time return to baseline.
Results:A total of 198 seizures (FLE was 115, TLE was 83) were included in this study. The baseline HR (in TLE, 74.9 ± 17.2 and in FLE, 70.7 ± 11.5 bpm), there was no difference between two groups. But the mean duration of the increased HR was more prolonged in TLE group (93.8 ± 54.9 seconds) than the FLE group(39.0 ± 21.4 seconds) (p < 0.001), the time to peak HR of the TLE group (135.1 ± 19.1 seconds) was higher than FLE group (119.3 ± 19.7 seconds) (p = 0.027), and the HR change of the TLE group (60.0 ± 26.3 bpm) was more prominent than that of the FLE group (22.8 ± 26.2 seconds) (p < 0.001). Furthermore, a longer duration of HR increase was seen in that of the TLE group than FLE group.
IntroductionHeart rate change is frequently seen in epileptic seizures, for example, ictal tachycardia and ictal bradycardia. Ictal tachycardia is defined as the occurrence of sinus tachycardia around the onset of ictal discharges.1 The prevalence of ictal tachycardia is very common(80–100%).2–5 It occurs more frequently in seizures with temporal lobe origin,6,7 especially with mesial temporal lobe onset.6,8 Ictal bradycardia is less frequent than ictal tachycardia; the prevalence has been calculated as ranging from 2.1% to 25.5%.4,9 It is most prevalent in seizures of the temporal lobe origin,9,10 and has a stronger association with bilateral hemispheric seizures.11
The heart rate (HR) is one of the most easy way to detect autonomic activity. The autonomic system (parasympathetic and sympathetic systems) has a major role to maintain homeostasis and regulate visceral functions such as HR.12 Epileptic seizures can cause changes in the autonomic functions affecting the sympathetic, parasympathetic, and enteric nervous systems.12 Epileptic seizure affects autonomic functions chemically or electrically.13 The anterior cingulate, insular, posterior orbito-frontal, and the pre-frontal cortices have important roles in regulating the autonomic nervous system at the cortical level; the amygdala, thalamus, and hypothalamus influencing autonomic nervous system at the subcortical level.1,14–16 In patients with epilepsy, the ictal discharges are propagated to these structures and the sympathetic or parasympathetic outflows are increased and this results in autonomic changes, such as a tachycardia.12 Tachycardia is earlier in right temporal lobe epilepsy (TLE) than left TLE because of right cerebral hemisphere is dominant in the sympathetic network.17
It is important extinguish from TLE to frontal lobe epilepsy (FLE). There are many studies comparing TLE and FLE. But those studies were focused on clinical semiology, such as the aura, automatisms, vocalization, and secondarily generalization or post-ictal symptoms. There were some studies about the HR change between TLE and extra temporal lobe epilepsy (XTLE),6,18,19 Adjei et al. investigated during subclinical electrographic seizures,18 but there has been no study about the difference of autonomic features, such as amount of change of HR, maximal HR, duration on HR change and so on, between TLE and FLE during clinical seizure - to the best of our knowledge. So, we compared the autonomic function or heart rate changes during seizures between FLE and TLE.
MethodsPatients and seizuresWe retrospectively reviewed the medical records of the Samsung Medical Center epilepsy monitoring unit (Seoul, South Korea) of those patients who had underwent long-term video EEG monitoring between January 2014 and April 2015. The inclusion criteria for this study were: (1) tachycardia, the HR exceed 100 bpm or increased 20% from baseline HR, (2) partial seizures without secondarily generalization, (3) scalp EEG seizures arising from the temporal or frontal regions, (4) identifiable QRS complexes on ictal ECG, and (5) more than 180 seconds of intervals with previous seizure.
The exclusion criteria for this study were: (1) any other diseases influencing autonomic system, (2) neurologic deficit, (3) ECG abnormality during the interictal period, (4) the seizures were emerged before 180 sec from previous seizure.
EEG and ECG recordingA scalp EEG was performed using the 10–20 system with additional frontotempral electrodes, simultaneous with ECG, right shoulder, left shoulder, A1, and A2 electrodes in an epilepsy monitoring unit (EMU). The data were recorded by the NicoletOne LTM system (Natus Medical Incorporated, Pleasanton, CA, USA) with a sampling rate of 512Hz. The EEG seizure onset was determined by three board certified neurologists.
Preictal-ictal-postictal HR changesThe HR was calculated form ECG R-R intervals or S-S intervals. The R-R intervals or S-S intervals were extracted by Microsoft Excel (MS Office 2010, Microsoft, USA). We obtained the ECG data during 180 seconds before the seizure onset, ictal period, and 180 seconds after the end of the seizure. The methods of this study can be seen in Figure 1. The “baseline HR” was determined by the mean HR from 120 seconds before a seizure onset to 90 seconds before a seizure onset. The “maximum HR” was determined by the biggest HR, after correction for the artifacts. The “seizure onset to peak” was defined as the time between the seizure onset and the time at maximum HR. The “HR change” was defined as the difference between the maximum HR and baseline HR. The “return to baseline” was defined as the time between the time of maximum HR and the time that the HR became smaller than the baseline HR after the maximum HR. The “return to baseline” were categorized as group 1; the return to baseline during ictal period as group 2; within 60 seconds after the ictal periods as group 3; between 60 seconds and 120 seconds after the ictal periods as group 4; and more than 120 seconds after ictal periods.
Statistical analysisThere was a comparison between FLE and TLE concerning the seizure duration, baseline HR, maximum HR, HR change, seizure onset to peak, and return to baseline. The statistical assessments were performed with the generalized estimating equation using SAS version 9.4 (SAS institute Inc, Cary, NC, USA)
ResultsFrom January, 2014 to April, 2015, there were 391 patients admitted at EMU. 51 patients had tachycardia. We selected 10 TLE, 10 FLE with matching sex. A total of 20 patients were enrolled in this study; 10 patients were men and 10 were women, aged 15 to 66 years. There were 10 patients who were FLE, with 10 as TLE. In total, 198 seizures were analyzed, 83 were TLE and 115 were FLE; with 87 seizures being right oriented, and 18 were left, 93 were bilateral. Table 1 shows the patients, seizure type, and onset. Figure 2 shows some typical examples of heart rate around the seizures in patients with TLE and FLE. The change of HR is prominent and prolonged in TLE than FLE. Table 2 shows the difference between FLE and TLE. The mean duration of seizures in TLE was 93.80 ± 54.92 seconds, whereas in FLE it was 39.0 ± 21.4 seconds, with a difference of 54.8 seconds (p < 0.001). The baseline HR was similar for the two groups: 74.9 ± 17.2 and 70.70 ± 11.5 bpm. The time to peak HR was 135.1 ± 19.1 seconds in the TLE group, whereas it was 119.3 ± 19.7 seconds in the FLE group, with a difference of 15.8 seconds (p = 0.027). The HR change was 60.0 ± 26.3 bpm in the TLE group, whereas it was 48.7 ± 18.5 bpm in the FLE group, with a difference of 11.3 seconds (p < 0.001). The mean duration of the seizure onset to peak was 47.8 ± 45.4 seconds in the TLE group, whereas it was 22.8 ± 26.2 seconds in the FLE group, with a difference of 25.0 (p < 0.001). Figure 3 shows the difference of the return to baseline between TLE and FLE groups. The seizures that returned to the baseline during the ictal period were 1 in the TLE group (1.2%) versus 9 in the FLE (7.83%) group. Before 60 seconds there were 19 in the TLE group (22.89%), whereas there were 62 in the FLE group (53.91%). Between 60 seconds and 120 seconds, there were 5 in the TLE group (6.02%), with 15 in the FLE group (13.04%). Over 120 seconds, there were 58 in the TLE (69.88%) group, whereas there were 29 in the FLE group (25.22%). Thus, in brief summary, in the TLE group, many seizures (69.88%) were returned to the baseline HR longer than 120 seconds, whereas in the FLE group, most (61.74%) were before 60 seconds. The p-value was 0.001.
DiscussionThe results of this study revealed that ictal tachycardia was observed in both the FLE and TLE group, but the HR change was different between TLE group and FLE group. We can suggest the reasons why TLE group has more prominent and prolonged than FLE group, the limbic system, which includes the amygdala and the hippocampus, and the insular cortex, are keys to the autonomic regulation in epilepsy. Tachycardia is more frequent in the TLE than other seizures, because of the involvement of the amygdala, hippocampus and right insular cortex.7 Many studies suggest that the propagation of epileptic discharges to the right insular cortex is a key part of the sympathetic-parasympathetic changes that influences the heart rate.20 Ictal tachycardia is more prominent in temporal lobe epilepsy (TLE),21 as compared with XTLE,9,22 which may suggest that the involvement of insular cortex is a key part of ictal tachycardia.1 The insular has a role in regulating autonomic nervous system.23 In TLE, compared with XTLE, the duration of the tachycardia is longer. This may be because epileptic discharges can propagate toward the insular cortex more easily and longer in TLE as compared with XTLE.1 There was tachycardia in FLE, the reason of tachycardia of FLE is due to orbitofrontal cortex.
Autonomic suprabulbar influences are mediated by several output pathways from orbitofrontal cortex to the thalamus, temporal lobe, amygdala, brain stem and so on.6 Stimulation of frontocortical-brain stem pathways influences sympathetic tone increase and may produce cardiac arrhythmias.24 Thus, the difference of HR between FLE and TLE was because of the involvement of the limbic system or orbitofrontal system. Compare TLE and FLE, the limbic system and insular cortex has more profound effect to the autonomic system than orbitofrontal cortex.
Tachycardia is one of the most important risk factor of sudden unexpected death in epilepsy (SUDEP).25 Tachycardia may be considered one of the key event in tachyarrhythmia and sympathetic over-activity. Tachyarrhythmia alter cardiac repolarization, and that make critical arrhythmia, and finally makes cardiac arrest.26 In TLE, more patient has higher risk of SUDEP.27 In our study, there are more prolonged and prominent HR change in TLE, and there are more sympathetic over-activity, and have more chance of cardiac arrhythmia, and so the patients might have higher risk of SUDEP.
The limitation of present study is the small number of patients, as well as the small numbers of FLE from the left hemisphere. We collected the data from an epilepsy monitoring unit, which estimated the benefit and possibility of epilepsy surgery. The left FLE has less chance to be operated on because of the presence of the language center in the left frontal lobe. Thus, very few left FLE patients and seizures are included in this study. Hence, more studies are needed to clarify the difference in HR pattern between FLE and TLE.
Other studies have been about ictal tachycardia, which focused on the start of the HR change and ictal onset. So, for example, they published which epileptic syndrome is earlier than another.17 Many studies analyzed the onset of HR change, seizure onset, and the correlation between them, but in this present study, there were many motion artifacts, thus we could not find the point to change the HR.
In TLE, the HR change is much prominent and prolonged than FLE, because of ictal discharge propagation to the limbic system and insular regions. Therefore, this difference can be an important point to differentiate between FLE and TLE.
Figure 1.Methods of analysis of the wave. The “baseline heart rate (HR)” was determined by the mean HR from 120 seconds before a seizure onset to 90 seconds before a seizure onset. The “maximum HR” was determined by the biggest HR, after correction for the artifacts. The “seizure onset to peak” was defined as the time between the seizure onset and the time at maximum HR. The “HR change” was defined as the difference between the maximum HR and baseline HR. The “return to baseline” was defined as the time between the time of maximum HR and the time that the HR became smaller than the baseline HR after the maximum HR. The “return to baseline” were categorized as group 1; the return to baseline during ictal period as group 2; within 60 seconds after the ictal periods as group 3; between 60 seconds and 120 seconds after the ictal periods as group 4; and more than 120 seconds after ictal periods. 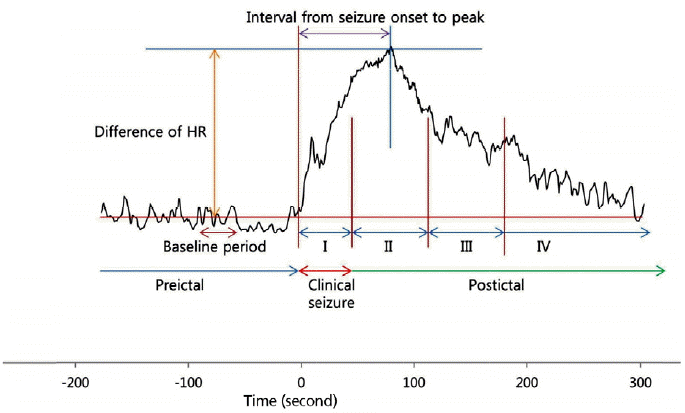
Figure 2.The typical examples of heart rate (HR) change in both group. (A) HR change in the typical temporal lobe epilepsy (TLE) case. (B) HR change in the typical frontal lobe epilepsy (FLE) case. In the TLE, the change is much prominent and prolonged than in that of the FLE. 
Figure 3.Return to baseline. Period 1 returned to baseline in ictal period, Period 2 did within 60 seconds, Period 3 did during 60 and 120 seconds, Period 4 did after 120 seconds. In the frontal lobe epilepsy (FLE), Period 2 was most frequent, but in the temporal lobe epilepsy (TLE), Period 4 was (p = 0.002). 
Table 1.The clinical characteristics of the patients Table 2.Comparison of the seizure and heart rate changes between the FLE and TLE groups References2. Marshall DW, Westmoreland BF, Sharbrough FW. Ictal tachycardia during temporal lobe seizures. Mayo Clin Proc. 1983;58:443–6.
3. Provini F, Plazzi G, Tinuper P, Vandi S, Lugaresi E, Montagna P. Nocturnal frontal lobe epilepsy. A clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122:Pt 6. 1017–31.
4. Rugg-Gunn FJ, Simister RJ, Squirrell M, Holdright DR, Duncan JS. Cardiac arrhythmias in focal epilepsy: a prospective long-term study. Lancet. 2004;364:2212–9.
5. Zijlmans M, Flanagan D, Gotman J. Heart rate changes and ECG abnormalities during epileptic seizures: prevalence and definition of an objective clinical sign. Epilepsia. 2002;43:847–54.
6. Garcia M, D’Giano C, Estellés S, Leiguarda R, Rabinowicz A. Ictal tachycardia: its discriminating potential between temporal and extra-temporal seizure foci. Seizure. 2001;10:415–9.
7. Schernthaner C, Lindinger G, Pötzelberger K, Zeiler K, Baumgartner C. Autonomic epilepsy--the influence of epileptic discharges on heart rate and rhythm. Wien Klin Wochenschr. 1999;111:392–401.
8. Leutmezer F, Schernthaner C, Lurger S, Pötzelberger K, Baumgartner C. Electrocardiographic changes at the onset of epileptic seizures. Epilepsia. 2003;44:348–54.
9. Galimberti CA, Marchioni E, Barzizza F, Manni R, Sartori I, Tartara A. Partial epileptic seizures of different origin variably affect cardiac rhythm. Epilepsia. 1996;37:742–7.
10. Tinuper P, Bisulli F, Cerullo A, et al. Ictal bradycardia in partial epileptic seizures: autonomic investigation in three cases and literature review. Brain. 2001;124:Pt 12. 2361–71.
11. Britton JW, Ghearing GR, Benarroch EE, Cascino GD. The ictal bradycardia syndrome: localization and lateralization. Epilepsia. 2006;47:737–44.
12. Eggleston KS, Olin BD, Fisher RS. Ictal tachycardia: the head-heart connection. Seizure. 2014;23:496–505.
13. Sevcencu C, Struijk JJ. Autonomic alterations and cardiac changes in epilepsy. Epilepsia. 2010;51:725–37.
14. Jeppesen J, Beniczky S, Johansen P, Sidenius P, Fuglsang-Frederiksen A. Detection of epileptic seizures with a modified heart rate variability algorithm based on Lorenz plot. Seizure. 2015;24:1–7.
15. Sévoz-Couche C, Comet MA, Bernard JF, Hamon M, Laguzzi R. Cardiac baroreflex facilitation evoked by hypothalamus and prefrontal cortex stimulation: role of the nucleus tractus solitarius 5-HT2A receptors. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1007–15.
16. Thornton JM, Aziz T, Schlugman D, Paterson DJ. Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans. J Physiol. 2002;539:Pt 2. 615–21.
17. Kato K, Jin K, Itabashi H, Iwasaki M, Kakisaka Y, Aoki M, et al. Earlier tachycardia onset in right than left mesial temporal lobe seizures. Neurology. 2014;83:1332–6.
18. Weil S, Arnold S, Eisensehr I, Noachtar S. Heart rate increase in otherwise subclinical seizures is different in temporal versus extra-temporal seizure onset: support for temporal lobe autonomic influence. Epileptic Disord. 2005;7:199–204.
19. Adjei P, Surges R, Scott CA, Kallis C, Shorvon S, Walker MC. Do subclinical electrographic seizure patterns affect heart rate and its variability? Epilepsy Res. 2009;87:281–5.
20. Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–32.
21. Epstein MA, Sperling MR, O’Connor MJ. Cardiac rhythm during temporal lobe seizures. Neurology. 1992;42:50–3.
23. Kahane P, Di Leo M, Hoffmann D, Munari C. Ictal bradycardia in a patient with a hypothalamic hamartoma: a stereo-EEG study. Epilepsia. 1999;40:522–7.
24. Zamrini EY, Meador KJ, Loring DW, et al. Unilateral cerebral inactivation produces differential left/right heart rate responses. Neurology. 1990;40:1408–11.
25. Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res. 2002;52:117–27.
|
|
||||||||||||||||||||||||||||||||||||