Introduction
Since the 19th century, it has been a controversial topic whether epilepsy is a progressive disorder. Repeated and continuous epileptic seizures that are generated from a primary focus can induce secondary epileptogenic zones in previously unaffected brain areas. This secondary epileptogenesis concept was first described by Morrel in animal studies.1 'Mirror focus' is a special form of secondary epileptogenesis; it means that the secondary epileptogenic zone is observed in a contralateral homotopic area to the primary epileptic focus.2 Some previous studies reported mirror focus for temporal lobe epilepsy with a tumor or focal lesion.3,4 We have observed a patient with mirror focus in left occipital lobe epilepsy.
Case
Noninvasive presurgical evaluation
A 10-year-old girl had epileptic seizures for 4 years. She was right handed and her epileptic seizures consisted of a visual aura with flashes, unresponsiveness and oro-ailimentary automatisms like lip smacking. The visual aura occurred in the left, right, or whole visual fields. Sometimes her seizures evolved into secondarily generalized seizures. Her seizures were not completely controlled by a polytherapy with carbamazepine, lamotrigine, and valproic acid. In a neurological examination, no lateralizing or focal neurological deficits were noted. She had no family history of epileptic seizure. Initially she was admitted to a long-term video EEG monitoring unit (EMU) for presurgical evaluation. During 9 day stay, interictal epileptileptifom discharges were seen frequently in the left occipital area (Fig. IA). Ictal events were 31 simple partial seizures (SPS) and 11 complex partial seizures (CPS). Among the 31 SPS, 12 visual auras occurred on her right side, whose ictal EEG showed left occipital discharges in 9 SPS and the remaining 3 auras showed no EEG changes. The other 19 visual auras occurred on her left side, whose ictal EEG revealed left occipital discharges in 2 SPS and the remaining 17 auras showed no EEG changes. Among the 11 CPS, right versive seizures are in 7 seizures, dialeptic seizures in 3 seizures, and left versive seizure in 1 seizure. Secondarily generalization occurred in 5 seizures. All ictal EEG changes started in the left occipital area (Fig. IB). Her 3.0T high resolution brain MRI showed no definite abnormalities. She underwent ictal and interictal Tc-99m ECD SPECT. Hyperperfusion was seen suspiciously in the left medial occipital area. The interictal SPECT showed hypoperfusion in the left medial occipital area. Substracted ictal SPECT co-registered to MRI (SISCOM) demonstrated a hyperperfused area in the left posterior temporal area. Brain 18F-FDG PET demonstrated bilateral posterior temporo-occipital hypometabolism, but the left temporo-occipital hypometabolism were more prominent than the right one. Functional MRI showed left language dominance. Those results of the first presurgical evaluation demonstrated the focal epilepsy was originated from left occipital area. And the neocortical area is in the dominant hemisphere and has no structural abnormalities. Invasive presurgical evaluation, after placement of subdural electrodes over the left temporo-occipital areas, was planned for resective epilepsy surgery.
Invasive presurgical evaluation and epilepsy surgery
She underwent subdural grids and strips around the left occipital area, and her seizures were recorded again in the EMU. She had 3 CPS and 34 SPS were recorded. The 3 CPS were right versive seizures and ictal EEG showed a start from the left occipital area. The 34 SPS included a visual aura. 19 visual auras occurred on her left side and none of them showed EEG changes. 15 visual auras occurred on her right side; for 2 of these auras, the EEG showed changes in the left occipital area, and the remaining 13 auras showed no EEG changes. Interictal epileptiform discharges were seen only in the left occipital areas. After the functional mapping using evoked potentials and brain stimulation, she has undergone neocortical resection of left occipital area including ictal onset zone. The pathology of the specimen demonstrated focal cortical dysplasia type I.
Postoperative follow-up EMU
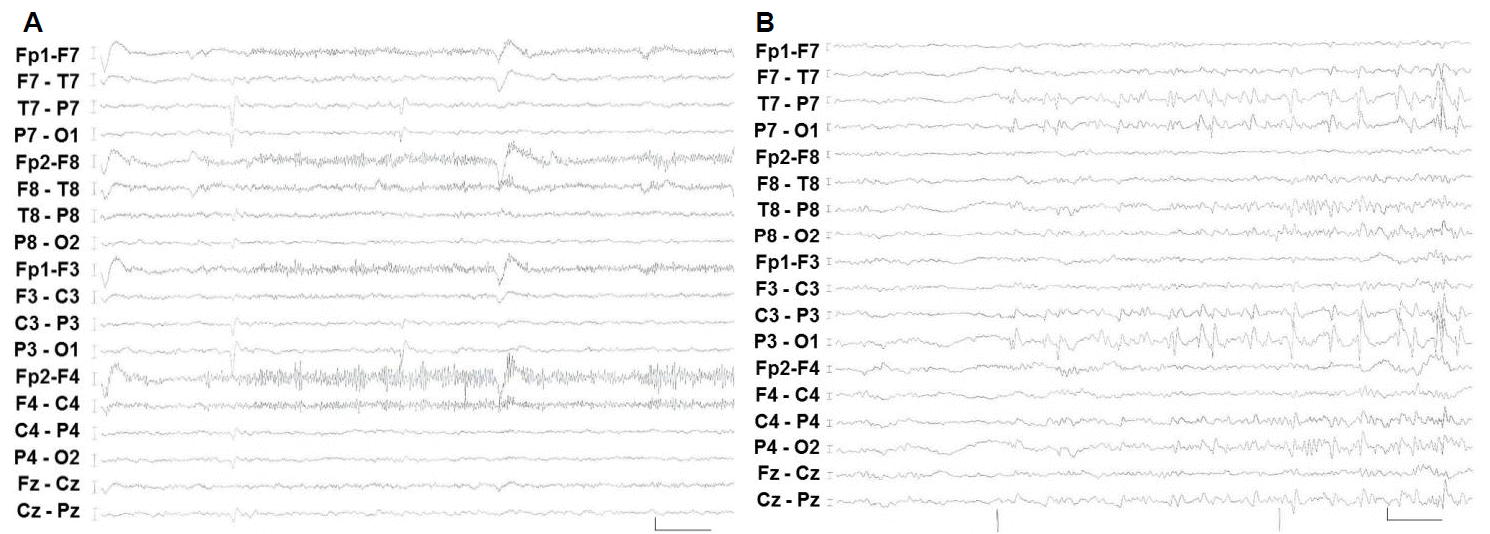
She had no seizures for 10 months after epilepsy surgery, but then her seizures started again with seizure frequency similar to that before the operation. She was re-evaluated by being admitted to EMU. In contrast to the first long-term video monitoring, interictal epileptiform discharges were seen every minute, all in the right occipital area (Fig. 2A). Ictal events were recorded in 17 seizures. Among them, 12 were SPS and 5 were CPS. All SPS consisted of visual auras that occurred on the left, right or whole fields. Among the SPS, electro-clinical correlations showed right occipital origin seizures were 1 out of 4 left visual auras, 0 out of 2 right visual auras, and 5 out of 6 whole field visual auras. But left occipital origin SPS were not seen. Among 5 CPS, 3 were left versive seizure and 2 dialeptic seizures. Generalized seizures were evolved from 3 out of 5 CPS. The ictal EEG of all CPS was originated from right occipital area (Fig. 2B). She also underwent ictal and interictal Tc-99m HMPAO SPECT. The ictal SPECT showed hyperperfusion in the right medial occipital area. SISCOM demonstrated a hyperperfused area in the right occipital areas (Fig. 2C). Brain 18F-FDG PET demonstrated left posterior temporo-occipital metabolism defects due to the previous operation. We concluded that the epilepsy classification was right occipital lobe epilepsy after epilepsy surgery, which was the mirror focus, compared with the previous left occipital focus.
Discussion
This is the case report about mirror focus in a patient with pharmacoresistant occipital lobe epilepsy with normal brain MRI. In the first study, our case’s seizure focus was left occipital lobe, based on the full presurgical evaluation including video EEG monitoring, interictal SPECT and 18F-FDG PET. Invasive presurgical evaluation and immediate surgical outcome demonstrated the left occipital focus. However, her epileptic seizures relapsed 10 months after the surgery. In the second study, the patient’s redeveloped focus was right occipital lobe, which is contralateral homotopic area, a mirror focus. There is a possibility that right occipital focus can be considered as the remained focus in case with initially bilateral occipital epilepsy. However, the clinical course and initial evaluation of definite unilateral occipital lobe epilepsy supports the development of mirror focus in the contra-lateral hemisphere.
To our knowledge, occipital lobe epilepsy with mirror focus is a rare condition. Most studies about mirror focus reported temporal lobe or frontal lobe epilepsy. 3–5 Only a small portion of cases involved extratemporal epilepsies. The mirror focus is not reported in occipital lobe epilepsy yet. We can suggest two reasons why the mirror focus is not reported in occipital lobe epilepsy. First, occipital lobe epilepsy is not common neocortical epilepsy. The frequency of occipital lobe epilepsy varies from 2% to 13% of symptomatic partial epilepsies.6,7 Second, the connection between left and right hemispheric homotopic area is not direct in occipital lobes. Frontal and parietal lobes are densely and closed connected via corpus callosum, and temporal lobes are via anterior and posterior commissure. But occipital lobes do not have direct monosynaptic connections between each lobe.
Morrell defined the three different stages of the secondary epileptogenesis process.8 The first stage is the dependent stage, in which interictal epileptiform discharges are observed synchronously with primary focus. The second stage is the intermediate stage, in which interictal epileptiform discharges and occasional seizures occur independent of the primary focus. Removal of the primary focus leads to cessation of seizure after a variable period. The third stage is the independent stage, in which interictal epileptiform discharges and seizures occur independent of the primary focus. Removal of the primary focus is followed by persistence of interictal epileptiform discharges and seizures. Our case showed the persistence of secondary interictal and ictal focus after removal of the primary focus. That means that the right occipital mirror focus in our case was in the third independent stage.
Goddard described the ‘kindling phenomenon (repeated stimulations lower the seizure threshold and induce more frequent seizures)’.9 Both mirror focus and kindling phenomenon are applied in animal models, but these ideas are disputed in human beings. These issues are very important in epilepsy patients, in order to decide on early aggressive medical treatments and plan epileptic surgeries. Usually occipital lobe epilepsy can have spread into frontal area through supra-Svlvian pathways and temporal lobe through intra-Sylvian pathway. Also our case suggests the possibility that contralateral spread can occur and make a mirror focus in contralateral homotopic area. If the formation of mirror focus is in occipital lobe epilepsy, it can cause fatal visual field dysfunction as well as progressive disabling seizures which cannot be surgically removed.
Our case may give an evidence of mirror focus even in occipital lobe epilepsy. Also the progression of epilepsy and timely surgery of focus should be deliberately considered in a patient with occipital lobe epilepsy.
Generally when we designate a lesion as a mirror focus in the third independent stage, interictal epileptiform discharges or seizures pesistently exist “before” and after resection of the primary focus. But any epileptiformdischarges or seizures of right occipital origin were not recorded in the long-term presurgical evaluation of this patient. Given that postoperative seizures unexpectedly developed from 10 months after epilepsy surgery, this was a latent mirror focus which was newly developed after the removal of the primary focus. It is necessary to classify a variety of mirror foci in the future.