A Case of Phenytoin-induced Rhabdomyolysis in Status Epilepticus
Article information
Abstract
Phenytoin is a commonly used antiepileptic drug, especially when treating status epilepticus. Here, we present a patient who suffered from status epilepticus and developed rhabdomyolysis after being treated with phenytoin. As multiple seizures itself can induce rhabdomyolysis, it is difficult to recognize that phenytoin can be the cause of rhabdomyolysis in status epilepticus patients. Even though phenytoin is a rare cause of rhabdomyolysis, we should discern that phenytoin can be a causative drug to bring about rhabdomyolysis.
Introduction
Phenytoin is one of the most commonly used anti-epileptic drugs for treating seizure disorders. The well-known adverse effects of phenytoin are nystagmus, ataxia, drowsiness as well as blood dyscrasia, nephrotoxicity, hepatotoxicity and hypersensitivity syndrome.1 Rhabdomyolysis by phenytoin was first reported at 1976 and has been rarely been reported since then.2 Here we present a patient with status epilepticus who suffered from rhabdomyolysis after being treated with intravenous (IV) phenytoin.
Case
A 37-year-old man visited the emergency center due to three events of generalized tonic-clonic seizures without recovery of consciousness between seizure from 30 minutes ago. Two-years ago the patient had left frontal intracranial hemorrhage (ICH) due to a ruptured aneurysm located at anterior communicating artery (Acom). The patients also had diabetes mellitus and liver cirrhosis due to chronic hepatitis B. The patient was receiving metformin 500 mg/day and linagliptine 5mg/day for diabetes and tenofovir 300 mg/day for hepatitis B.
On the presentation to emergency center, the blood pressure was 125/55 mmHg, the heart rate was 112/min, and the body temperature was 37.2°C. The patient was in coma with intact brainstem reflexes. There was no lateralizing sign or any other focal neurological deficit except myoclonic jerks were observed from the chest and abdominal wall continuously. The patient was intubated and IV lorazepam 4 mg was injected twice. After then, phenytoin 20 mg/kg was loaded with starting 24 hr electroencephalography (EEG) monitoring. The myoclonic jerks subsided after phenytoin loading. Brain computed tomography (CT) demonstrated an encephalomalacia at the left frontal lobe due to the prior ICH (Fig. 1). In initial laboratory tests, the creatine kinase (CK) was elevated to 727 IU/L, but the estimated glomerular filtration rate (eGFR), blood urea nitrogen (BUN) and creatinine level was in the normal range (eGFR: 70 mL/min/1.73 m2, BUN: 13 mg/dL, Creatinine 1.31 mg/dL). The patient became alert and no more clinical seizure was observed after phenytoin treatment. The 24hr EEG monitoring showed continuous medium amplitude theta to delta slowing on the left hemisphere due to the encephalomalacia without any epileptiform discharges.

Brain computed tomography (CT) showed the encephalomalacia in the left frontal lobe due to previous intracranial hemorrhage (ICH) and clipping at the anterior communicating artery.
Since the level of CK and creatinine increased to 1,823 IU/L and 2.93 mg/dL at the second day of hospitalization, massive hydration with bicarbonate therapy initiated to treat acute kidney injury. Oral phenytoin 150 mg twice a day was maintained to control seizure. Serum CK decreased to 824 IU/L transiently after starting hydration, but it increased again at the 6th hospital day. Even though aggressive hydration was performed and no more seizures were observed, the serum CK levels peaked at 3,825 IU/L at the 7th hospital day. Considering that phenytoin might be the cause of rhabdomyolysis, phenytoin was substituted with levetiracetam at the 7th hospital day. Subsequently, the serum CK level promptly trended to decrease and was normalized at day 13 (Fig. 2). No more seizures were observed, and the patient was discharged to home at day 13.
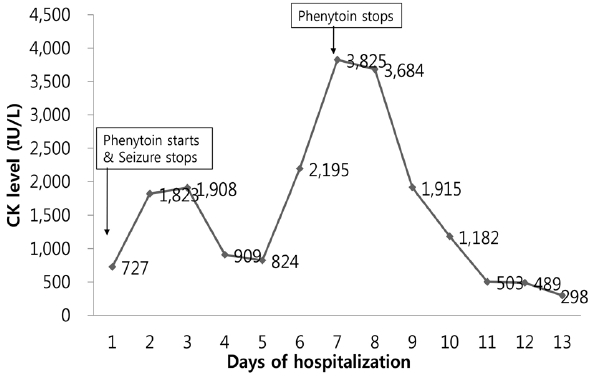
Creatine kinase (CK) levels with time. The level of CK increased up to 1,908 IU/L on the 3rd day after the last seizure and it decreased to 824 IU/L on the 5th day. But, it increased again to 3,825 IU/L even though there were no more seizures. After discontinuing the phenytoin at the 7th day, the level of CK immediately decreased.
Discussion
In the present case, the level of CK increased to 1,908 IU/L at the 3rd day after last seizure and decreased to 824 IU/L at the 5th day after last seizure and increased again up to 3,825 IU/L for the two consecutive days, although there were no additional seizures, immobilizations or any traumas. During hospitalization, the patient received baclofen for hiccups, tenofovir for hepatitis B and linagliptin for diabetes mellitus. But these drugs are not known to cause rhabdomyolysis. The level of CK immediately decreased after discontinuing phenytoin, and consistently decreased to 298 IU/L at the 6th day after stopping phenytoin. In the previous study about postictal CK elevation, the peak level was observed at 2–4 days after last seizure.3 Therefore, in the present case the second rise of CK level at the 7th day after last seizure could not be explained by the seizure itself and the CK level was normalized after phenytoin discontinuation. We diagnosed the cause of the second rise of the CK level as rhabdomyolysis by phenytoin. Phenytoin was not re-administrated to confirm our hypothesis due to ethical problems.
In the literature review, the classic phenytoin-induced rhabdomyolysis was associated with phenytoin hypersensitivity syndrome, which is a reaction that typically develops within three weeks to three months after initiation of phenytoin medication.4–8 Phenytoin hypersensitivity syndrome is characterized by fever, rash, lymphadenopathy, and eosinophilia. In the present case, the absolute eosinophil count was in normal range, and other presentations of phenytoin hypersensitivity syndrome were not observed. Recently, the cases of phenytoin-induced rhabdomyolysis without any distinct symptoms of hypersensitivity have been reported1,9, and those cases were very similar to our patient. They suffered from generalized tonic-clonic seizures and were treated with IV phenytoin. The serum CK level increased up to the highest level at the 5th day after last seizure and promptly decreased after stopping phenytoin. This temporal correlation provides significant evidence of phenytoin-induced rhabdomyolysis. Our patient accords with the latter type of rhabdomyolysis.
Considering the wide use of phenytoin, the reports of phenytoin-induced rhabdomyolysis are very rare. There can be several causes to explain this. First, the rhabdomyolysis can be caused by status epilepticus itself, it may be hard to distinguish the exact cause of rhabdomyolysis in certain cases, especially, when the CK level fluctuates after multiple seizures. Second, the rise of the CK level by phenytoin is mild and transient than rhabdomyolysis which is caused by multiple seizures.
Rhabdomyolysis is a serious complication of phenytoin and leads to acute kidney injury. So, we should consider that phenytoin can be a causative drug of rhabdomyolysis especially when the CK level increases although the seizure is well-controlled by phenytoin therapy.