Predictors of Seizure Recurrence in Solitary Calcified Neurocysticercosis in Relation to Computed Tomography Scan: Prospective Observational Study
Article information
Abstract
Background and Purpose
Solitary calcified neurocysticercosis (NCC) on the computed tomography (CT) scan of brain in patients of epilepsy is common finding in endemic regions. Factors causing seizures in such cases are debatable. Immature calcification may be the causative factor for seizure recurrence. Thus, we aimed to study predictors of seizure recurrence specific to morphological characteristics on CT scan.
Methods
Patients with solitary calcified NCC on CT scan brain and active seizures were prospectively included. The protocol included clinical evaluation, contrast-enhanced CT scan of the brain, and electroencephalogram (EEG) at baseline and 9th month of 1-year follow-up in all patients. Seizure recurrence after 1 week of enrolment was recorded.
Results
One hundred twenty patients with a mean age of 23.33±12.81 years were included with a final follow-up of 109 patients and 35 patients had seizure recurrence. On univariate analysis, seizure frequency of more than 1 episode/month (45.7% vs. 25.7%, p=0.037; odds ratio [OR], 2.06; 95% confidence interval [CI], 1.05–5.68), perilesional edema on CT head (45% vs. 10.8%, p<0.001; OR, 6.95; 95% CI, 2.58–18.7), lower density (HU) of lesion on CT head (139.85±76.54 vs. 204.67±135.9 HU p=0.009) and abnormal EEG at presentation (p<0.001; OR, 18.25; 95% CI, 2.15–155.13) were significantly associated with seizure recurrence. On multivariate analysis, presence of perilesional edema on CT head (p=0.001; OR, 6.854; 95% CI, 2.26–20.77), density of lesion on CT (HU) (p=0.036; OR, 0.995; 95% CI, 0.99–1) and abnormal EEG (p=0.029; OR, 12.125; 95% CI, 1.29–113.74) were independently associated with seizure recurrence.
Conclusions
The presence of perilesional edema, HU of calcification on CT brain, and abnormal EEG suggest an increased risk of seizure recurrence in patients of epilepsy with solitary calcified NCC.
Introduction
Neurocysticercosis (NCC) accounts for 29% of epilepsy world-wide.1,2 Calcified granuloma is the final stage of the parenchymal cysticercus larva. It happens to be the most common radiological finding in the endemic area (10–20% of the endemic population).3 Previously, calcified NCC was supposed to be inert, but recent studies suggest that this calcified granuloma can be epileptogenic. Roughly one-third of the patients with calcified NCC have seizures4 and up to 34% of cases can show seizure recurrence.5 The factors responsible for epilepsy in the case of a calcified granuloma are debatable; however, status epilepticus at presentation, serial seizures, perilesional edema, perilesional gliosis, and larger size of the lesion have been suggested to be predictors of seizure recurrence.5,6 Blood-brain barrier dysfunction and hippocampal sclerosis secondary to calcified granuloma may be responsible for seizure recurrence, too.3,7,8 Patients of calcified NCC with seizures are usually controlled with anti-epileptic drugs, but a longer duration of treatment may be required due to seizure recurrence.7,9,10 Rarely, calcified NCC can be the cause of drug-resistant and surgically remediable epilepsy with better outcomes after surgery.11
We hypothesized that solitary calcified granuloma with less density of calcification (immature calcification) has a greater propensity to cause a recurrent seizure. Therefore, we planned to study it in detail like quantifying the density of calcified NCC on computed tomography (CT) scan and correlation with seizure recurrence in addition to other morphological characteristics of the calcified granuloma. Clinical and electroencephalographic features were also studied for any association. Our study is different from previous studies as most of the previous studies had used magnetic resonance imaging (MRI) brain.
Methods
This was a prospective observational study performed in the Neurology department, in association with the department of Radio-diagnosis at King George’s Medical University, Lucknow, India. The institutional review board gave ethical approval. Informed written consent was taken from all the patients. Subjects were enrolled from December 2017 to February 2019 and followed up for one year. Any case with solitary intracranial parenchymal calcification and seizures was enrolled. A single round solitary calcification on CT scan was defined as calcified NCC since such lesion is considered as calcified NCC in the endemic area until proven otherwise.12 Patients with other lesions along with solitary calcification (NCC in other stages), neuroimaging suggestive of an alternative diagnosis, family history of seizures, multiple seizure types, nonepileptic events, and refusal to give consent were excluded. Seizure recurrence was defined as the recurrence of a seizure 1 week after enrollment in the study. Detailed clinical history and physical and neurological examinations were performed in all subjects. Seizure duration, semiology of seizure, postictal deficits, impairment of consciousness, occupational habits, and residence were recorded from every patient. We followed the guidelines of the International League Against Epilepsy (ILAE) for the classification of seizures.13 The socioeconomic status of the patients was classified by using Modified Kuppuswamyscale.14
Patients were separated into two groups, new-onset (Naïve) and chronic epilepsy. A new-onset seizure group was defined as a seizure onset less than 1-year duration. Biochemical investigation including liver and renal function tests were performed in all patients. Serological tests for NCC were not done as its utility is doubtful in calcified NCC. A 64-slice contrast-enhanced CT head (Philips BrillianceTM, Thermo Fisher Scientific, Waltham, MA, USA) was done at baseline and 9 months follow-up. The scans of 2 mm thickness were acquired before and after giving intravenous injection of Iohexol at a dose of 1.5 mL/kg. All CT scans were analyzed by an expert radiologist who was not blinded to the study. Perilesional hypodensity on a CT scan was defined as perilesional edema. Persistence of perilesional hypodensity on follow-up scan at 9 months was defined as perilesional gliosis.15 Quantification of calcification of calcified NCC was done in terms of density, size, and volume on non-contrast CT head by using Radiant Dicom software. In this software, first, we opened the CT images, and then selected the calcified lesion. Using an elliptical/circular marker, we marked the calcified lesion in each slice of the CT scan, and then the area and density of calcification were calculated by the software. The slice with the maximal area and density was taken as the value for the analysis. Volume was estimated by the formula ABC/2 based on the ellipsoid volume equation, where A=maximum length-(in cm), B=width perpendicular to A-on the same head CT slice, and C=the number of slices multiplied by the thickness of slice.16
Electroencephalography (EEG) of 30-minute duration was done on every patient enrolled in the study. EEG recording was done with 26 electrodes (including 6 inferior temporal and 1 ECG) on the Nicolet V44 machine (Natus, USA) and the electrodes were placed following the standard 10–20 system. EEG was classified into 2 groups, normal or abnormal, and defined as abnormal if spikes, sharp waves, poly-spike or spike, and slow waves were seen. All the patients were continued on standard antiepileptic drugs if seizures were controlled. Oxcarbazepine as first-line and clobazam as the first add-on was used on patients who were not on treatment. Levetiracetam was used as a substitution for oxcarbazepine whenever needed. They were followed every 3 months and clinical features were recorded. CT scan and EEG were repeated at 9 months.
Statistical analysis
The statistical analysis was performed by using IBM SPSS Statistics Version 24.0. The categorical variables were expressed as proportions. The continuous variables were expressed as mean±standard deviation and Median plus Interquartile range. The categorical variables among seizure recurrence and non-recurrence groups were compared using Fisher exact test and chi-square test. The continuous variables were compared using the unpaired t-test. The paired continuous variables and paired categorical variables were compared using the Wilcoxon Sign Rank Sum test the McNemar tests, respectively.
All p-values<0.05 were taken as significant. Predictors of seizure recurrence were assessed with a multivariate regression model by using binary Logistic Regression. The time to seizure recurrence analysis was performed by using the Kaplan Meier Curves/log Rank test. Furthermore, a multivariate Cox-Proportional Hazard Model was derived to predict time to seizure recurrence.
Results
The mean age of the patients was 23.33±12.81 years (range 7–89 years) and 71 (59.2%) patients were males. Eighty-eight (73.3%) patients were residents of the rural area and 80 (66.67%) belonged to the lower middle class. Baseline clinical, EEG, and neuroimaging characteristics have been summarized in Supplementary Table 1. Mean density, volume, and length of calcification were 159.22±172.46 HU, 0.128±0.192 cm3, and 5.33±2.23 mm, respectively. Perilesional edema was seen in 24 cases (20%) and contrast enhancement in 3 cases (2.5%). The new-onset seizure was present in 22 (18.3%), 41 (34.2%) had seizure frequency more than 1 per month, and 8 had (6.7%) abnormal EEG. Out of 120 patients 11 were lost to follow up, so finally data from 109 patients were analyzed (Supplementary Fig. 1). It showed that 35 (32.11%) had seizure recurrence, the mean density of lesion was 230.20±153.86 HU, mean volume 0.997±0.17 cm3, mean length 4.62±2.05 mm, and none had perilesional edema or contrast enhancement on CT scan. EEG abnormality was persistent in 4 (3.7%) cases (Table 1).
On comparing from features at baseline, follow-up showed that changes in density, volume, and perilesional edema were significant. Other significant changes were the number of patients with a frequency of seizure more than 1 per month and having a headache, being 0, and 9.2%, respectively (Table 1). Two subgroups, seizure-recurrence (n=35) and seizure-free (n=74), had also shown similar significant changes from baseline except for calcification volume in the seizure recurrence group (Table 2). Furthermore, univariate analysis to compare all baseline parameters between seizure recurrence and seizure-free groups showed that significant parameters were seizure frequency >1/month (45.7% vs. 25.7%, p=0.037; OR, 2.06; 95% CI, 1.05–5.68), perilesional edema (45.7% vs. 10.8%, p≤0.001; OR, 6.95; 95% CI, 2.58–18.7), lesser density of the lesion (139.85±76.54 vs. 204.67±135.9 HU, p=0.009, vector autoregression, 4.83; 95% CI, 15.92–113.73), and abnormal EEG (20% vs. 1.4%, p≤0.001; OR, 18.25; 95% CI, 2.15–155.13) (Supplementary Table 2). Binary logistic regression considering seizure recurrence as dependent variable showed that presence of perilesional edema on CT (p=0.001; OR, 6.854; 95% CI, 2.26–20.77), density of lesion on CT (HU) (p=0.036; OR, 0.995; 95% CI, 0.99–1) and abnormal EEG (p=0.029; OR, 12.125; 95% CI, 1.29–113.74) were the factors independently associated with seizure recurrence (Table 3).
The final model derived from these factors along with headache showed good discrimination as evident by AUC=80.9% (95% CI, 0.72–0.90) and good calibration as evident by Homster and Lemeshow test (p=0.743) (Fig. 1). Kaplan-Meyer analysis found a significant difference in the time to event (seizure) when the patients were categorized according to the frequency of seizure ≥1 episode/ month (Log-rank test p=0.036) and perilesional edema on CT (Fig. 2, Log-rank test p<0.001). The seizure recurrence rate was significantly higher in patients with frequent seizures at baseline and perilesional edema on CT Head. We have also looked at sub-lobar localization of the lesions and any association with seizure recurrence, but results were negative except that mesial frontal calcification were found to be just significant in terms of fewer chances of being associated with seizure recurrence (Supplementary Table 3).
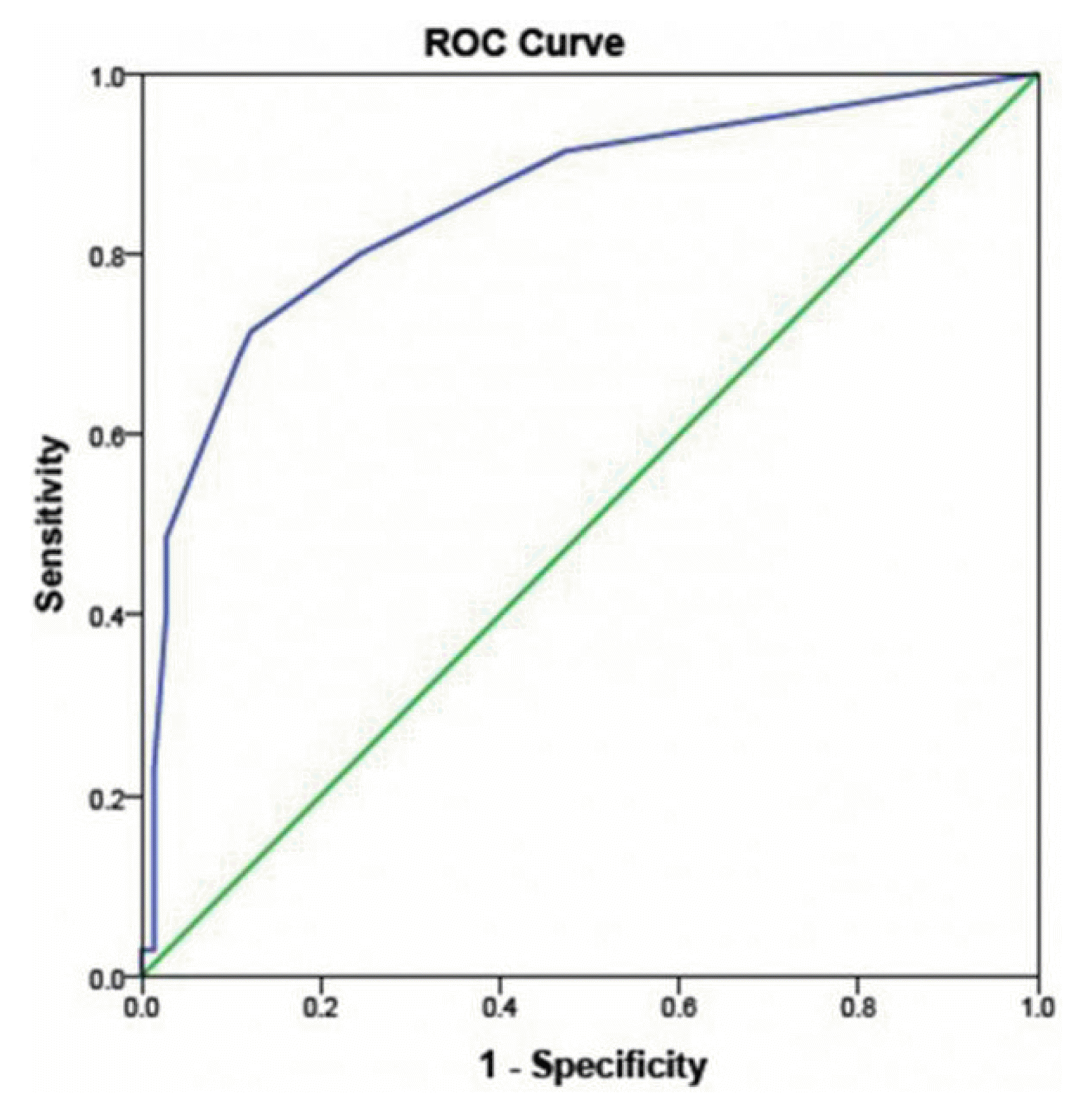
ROC curve derived from clinical, radiological, and electrophysiological parameters (area under the curve, 85.5%; Homster and Lemeshow test, p=0.58). ROC, receiver operating characteristic.
Discussion
Neurocysticercus ends up with solitary calcification in 35–40% of cases in its natural history17 and now it is increasingly being supported with evidence about its epileptogenicity.7,8 This study found that 32.11% (35/109) cases with epilepsy and solitary calcified NCC had seizure recurrence over one year follow up. We had used stringent criteria to avoid nonepileptic attacks and other epilepsies and found that the proportions of patients with seizure recurrence are like the previous studies.5,18 Our center has been commonly seeing NCC in its various stages of natural history. It was our observation that a lower density of calcification was associated with seizure recurrence, so we wished to study this aspect prospectively. It was a novel idea and our results have shown that lower density has an association with seizure recurrence, which is being reported for the first time.
There was an increase in calcification density from baseline to final follow up which is expected as the lesion matures. The quantification parameters like volume and size had decreased at the final follow-up, showing that with the maturation calcification shrinks due to dehydration. The perilesional edema on the CT head was an independent predictor of seizure recurrence like in previous studies.4,5,17 The perilesional edema is supposed to be the transient response of the host to released antigens of parasite from the calcified lesion.5,7 We did not find any case of perilesional gliosis in our study as all the cases, who had perilesional hypodensity on CT scan, showed complete disappearance at repeat CT head at 9 months (Fig. 3). This finding contrasts with previous studies, but those were MRI-based studies and MRI is more sensitive in picking up persistent gliotic changes.15 Although we had done an MRI brain with contrast in all cases at baseline, it was not repeated at follow-up due to its cost. The baseline MRI data did not add much to what we had from the CT scan. The location of the lesion was commonest in the frontal lobe like the study by Singh et al.18, but the lobar or sub-lobar location of the lesion was not associated with any increased seizure recurrence risk in our study. This probability may be explored with a larger sample size as we had only two cases of calcified granuloma in orbitofrontal and mesial temporal regions, which are supposed to be highly epileptogenic; however, this finding might explain easily controllable seizures and infrequent drug-refractory epilepsy even in such patients.
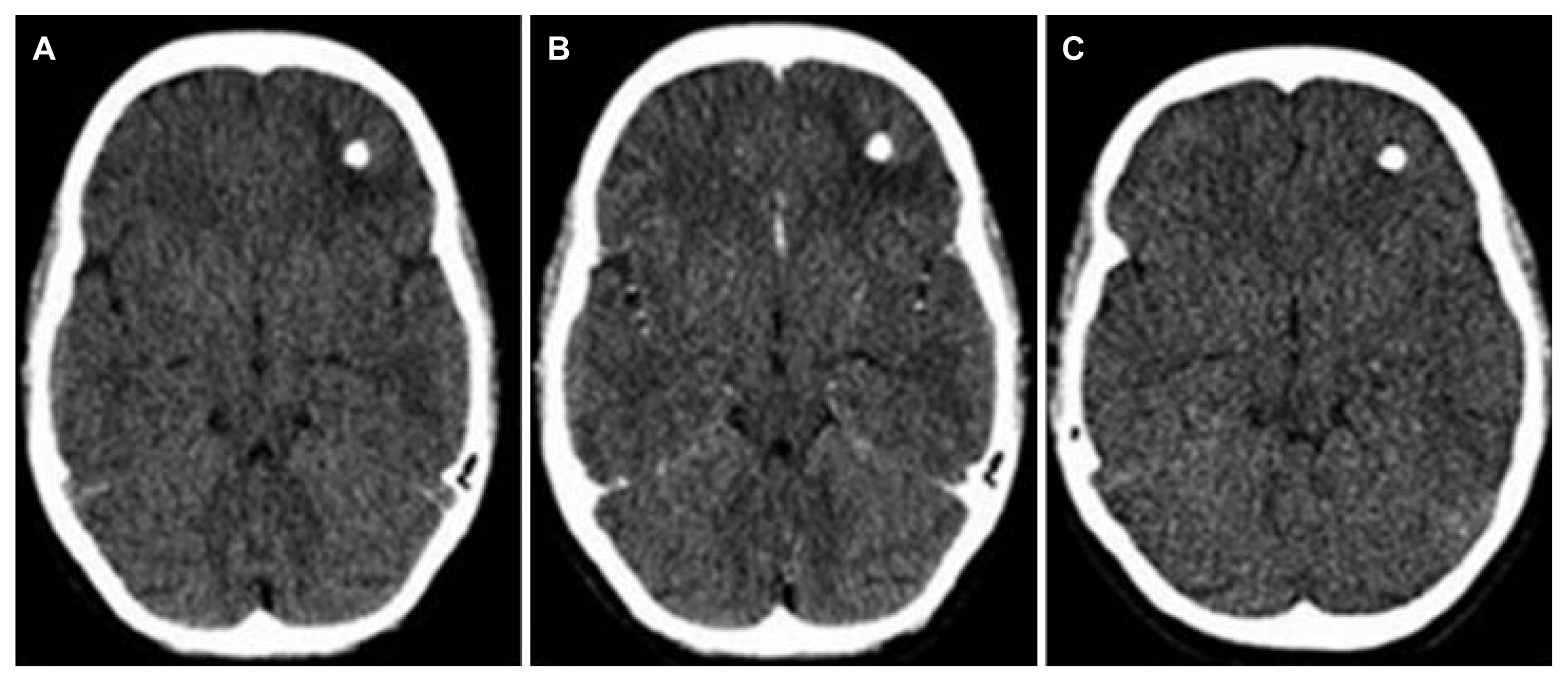
The computed tomography scan brain. (A) Left frontal calcified granuloma with perilesional hypodensity in plain scan. (B) Scan after contrast showing no enhancement. (C) Follow up scan at 9 months showing complete resolution of perilesional hypodensity.
Our findings suggest that calcified NCC may not be an inactive or inert lesion like a few other studies.19–21 This study showed that high seizure frequency at presentation was the only clinical variable that was significantly associated with the risk of seizure recurrence. This finding is like the studies wherein serial seizures and Todd’s palsy was significantly associated with seizure recurrence.5 We did not observe the same high seizure frequency in patients who had seizure recurrence on follow-up, and this could be due to ensuring appropriate drug treatment and compliance. The EEG abnormality was found only in 8 cases (7.3%) at baseline and 7 out of them were in the seizure recurrence group. This was again an independent predictor of seizure recurrence, which was like the previous studies.5,9 We know that the overall yield of interictal EEG is 15–50% which can further be increased by 15–35% by sleep deprivation, serial EEGs, or doing EEG within 24 hours of EEG.22 Factors like one-time EEG without sleep deprivation, irrespective of seizure timing, and episodic antigen release mediated inflammation leading to seizure development by an otherwise inert lesion could be possible reasons for the low yield of EEG in our study. Previously, another study has also shown about 8% cases with EEG abnormality even in patients with active lesion of cysticercosis from India.23
We studied CT characteristics of the lesion in terms of its size, volume, and density after defining the region of interest using freely available Radiant View Software. Although our method may appear crude, we found that calcification of lesser density is an important predictor of seizure recurrence, and it is a novel finding which can further be explored by using better methods. As earlier discussed, there is evidence to suggest that solitary calcified granuloma may be epileptogenic, but the reasons are unclear at present. The incomplete consolidation of calcifying granuloma or intermingling of cysticercus antigen within calcification leading to intermittent antigen release followed by transient inflammation, perilesional edema, and development of seizures have been postulated as possible mechanisms.24 Thus, immature calcification has a higher propensity to cause seizures owing to more chances of antigen-mediated inflammation. The presence of scolex within the calcified granuloma has also been found to have an increased risk of antigen-mediated inflammatory reaction and in turn seizures.25 The deleterious inflammation at consolidation or degenerating phase may cause persistent changes around calcified granuloma and pose a future risk of seizure as it is seen with perilesional gliosis. One year of follow-up is too short to comment upon seizure recurrence risk in the long term, and thus further follow-up may answer this question. Another limitation of our study was not using follow-up MRI for better assessment of perilesional gliosis.26 Moreover, we had not repeated the CT head at the time of seizure recurrence so we cannot comment about the nature of the lesion during that time. In conclusion, the lesser density of calcification and perilesional edema on CT scan of the brain, frequent seizure at presentation, and abnormal EEG are significant predictors of seizure recurrence in patients of epilepsy with solitary calcified NCC.
Supplementary Data
Plan of study. NCC, neurocysticercosis; CT, computed tomography; EEG, electroencephalogram; MRI, magnetic resonance imaging; CECT, contrast enhanced computed tomography.
Baseline characteristics (n=120)
Comparison of characteristics between seizure recurrence and seizure-free groups
Seizure recurrence risk with sub lobar localization of calcification
Notes
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Financial Support
This study was conducted using resources of the university. No external financial support was required.
Acknowledgement
We acknowledge our institution and its research cell for the necessary financial support to conduct this study. All the authors contributed equally.



