Cefepime- Induced Non-Convulsive Status Epilepticus (NCSE)
Article information
Abstract
Cefepime is a fourth-generation B-lactam cephalosporin, commonly used in immunosuppressed patients. Neurotoxicity, which present as nonconvulsive status epilepticus (NCSE), has been reported previously especially in adult patients with impaired renal function. We present a case of cefepime induced NCSE after recovering from acute renal failure. A 71-year-old woman was hospitalized for right lower lobe lobectomy after diagnosis of lung cancer. Although she had successful lobectomy, she underwent several post operative complication including operation site bleeding, acute renal failure, acute respiratory distress syndrome, and atypical pneumonia. Her renal failure was prerenal type after massive operation site bleeding, and continuous renal replacement therapy (CRRT) were started for renal replacement treatment. After 5 days of renal replacement therapy, her serum creatinine level was much improved from 2.7 mg/dL to 1.33 mg/dL. Cefepime renal dose were started, when atypical pneumonia became resistant to imipenem and vancomycin. After 5th day of cefepime use, the patient became stupor and developed one episode of brief generalized myoclonic seizure. Her electroencephalograph (EEG) revealed 2–3 Hz generalized sharp and with impression of NCSE, she was started on anti-epileptic treatment. Clinical symptoms improved 3 days after discontinuation of cefepime. She was than diagnosed with cefepime induced non convulsive status epilepticus. Anti-epileptic treatments were than discontinued uneventfully. Awareness of the potential neurotoxic clinical manifestations of various antibiotics and high degree of vigilance in critically ill patients is essential in identifying a potentially serious though reversible complication of antibiotic therapy.
Introduction
Antibiotics are among the most frequently used pharmaceuticals in both the inpatient and outpatient setting. Cephalosporins are grouped in B-lactam class of antibiotics, along with penicillins and carbapenems. While these antimicrobial agents are generally well tolerated, these drugs are not without their associated side effects, and neurotoxic effects are perhaps much less recognized. Neurotoxicity of cephalosporins has been reported with first generation cephalosporins such as cefazolin, second generation such as cefuroxime, third generation such as ceftazidime and fourth generation such as cefepime and can range from slurred speech, tremor, seizures and NCSE.1 The threshold of neurotoxicity is decreased in settings of reduced creatinine clearance, impaired renal function, pre-existing CNS conditions, and with use of third and fourth generation cephalosporines.2 NCSE is a conditions in which electrographic seizure activity is prolonged and results in non-convulsive clinical symptoms, usually lasting >30 min.3 Cephalosporines, particularly cefepime, have been associated with NCSE, especially in adult patients with impaired renal function. We present a case of 71-year-old women who developed NCSE after use of cefepime after recovering from acute renal failure.
Case report
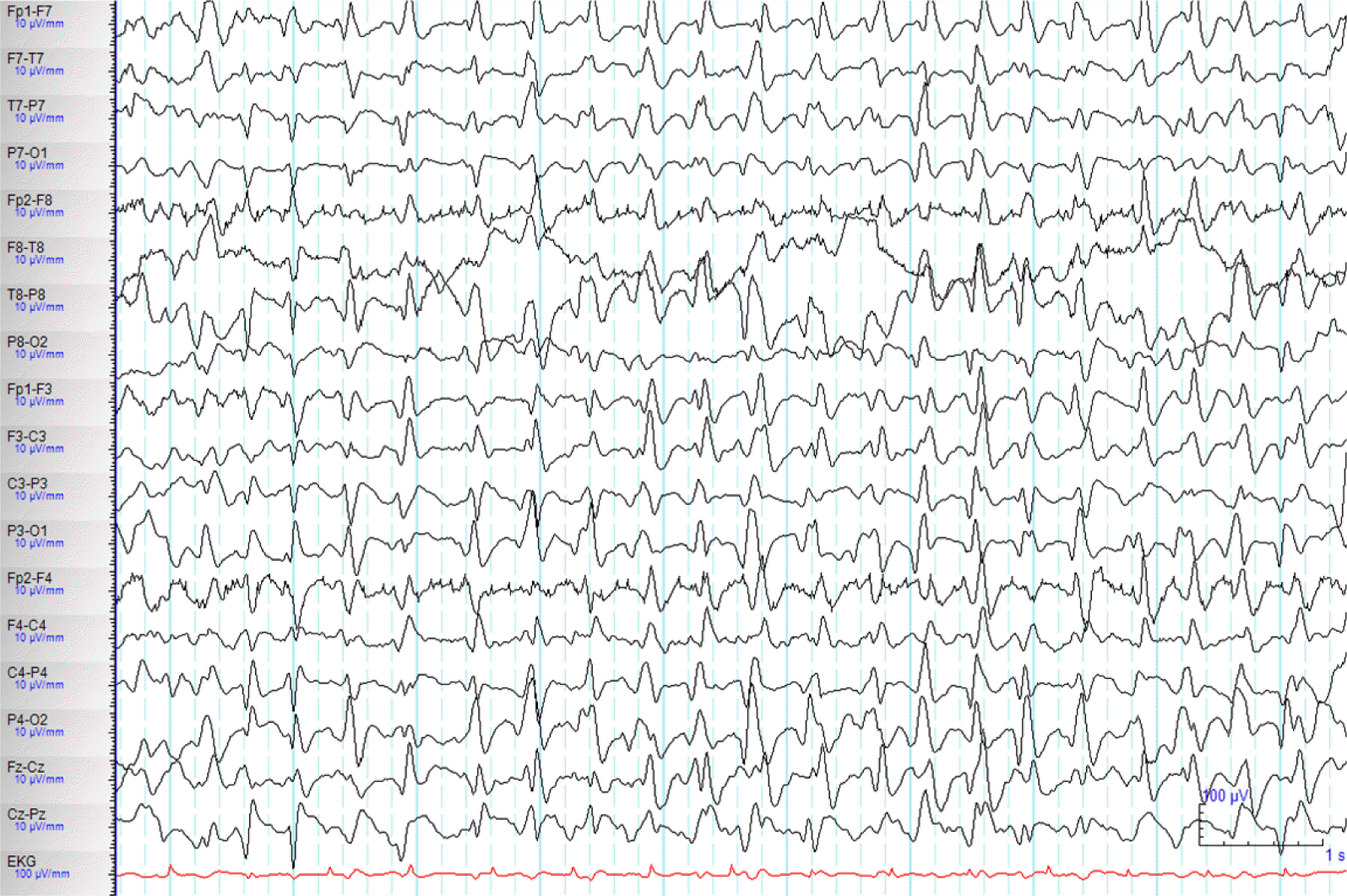
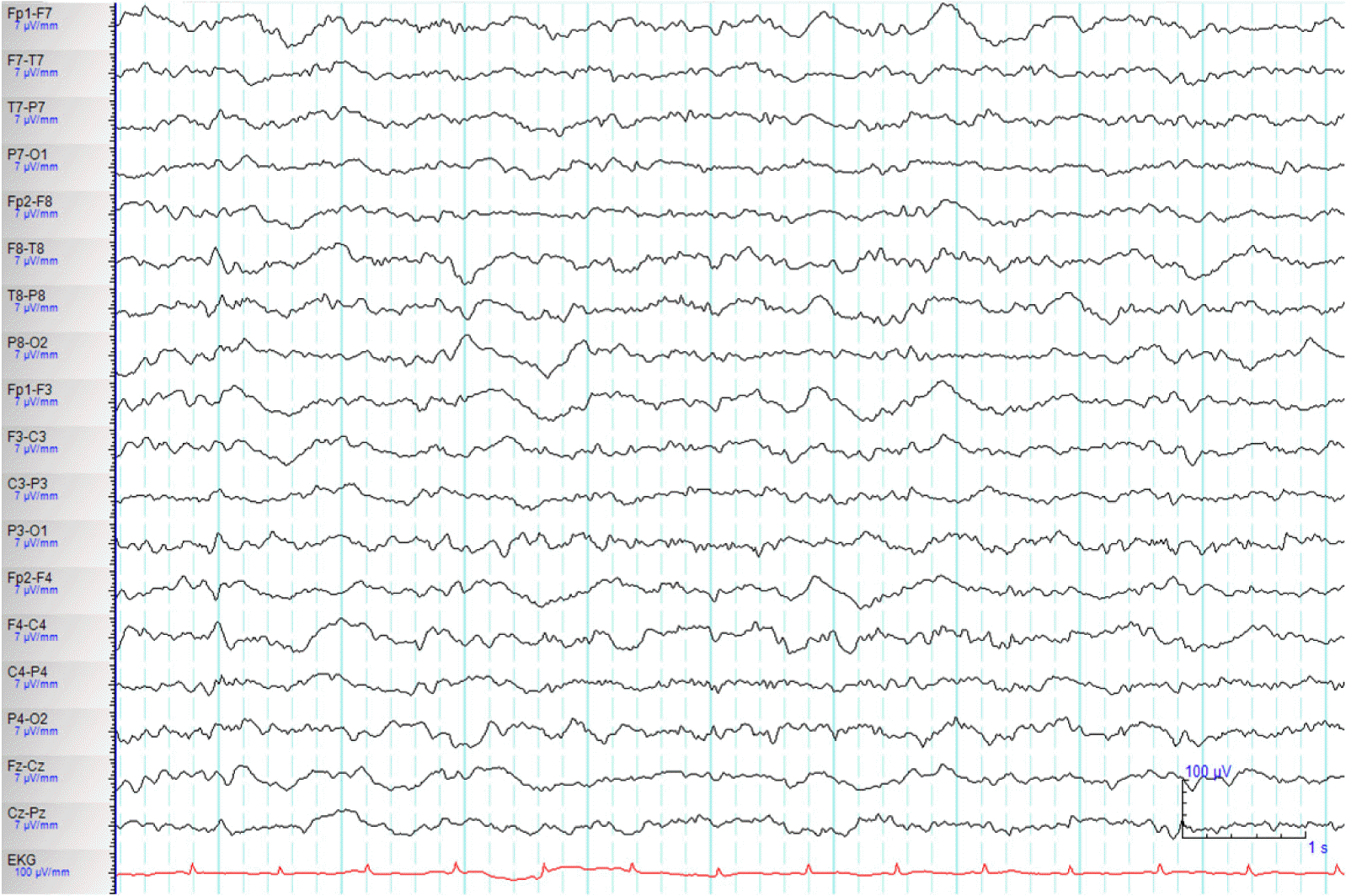
A 71-year-old woman with a history of diabetes mellitus and liver cirrhosis associated with hepatitis B viral infection was hospitalized for right lower lobe lobectomy after diagnosis of lung cancer. Although she had successful lobectomy, she underwent several post-operative complication including operation site bleeding, acute renal failure, acute respiratory distress syndrome, and atypical pneumonia. Her renal failure was prerenal type after massive operation site bleeding, and continuous renal replacement therapy (CRRT) were started for renal replacement treatment. Post operation (OP) day 9, she experienced confusion, and disorientation and started on quetiapine with diagnoses of delirium. Post OP day 13, she had antibiotic resistance for atypical pneumonia, which had to switch from imipenem and vancomycin to cefepime. Although her serum creatinine level was much improved from 2.7 mg/dL to 1.87 mg/dL, after 5 days of renal replacement therapy, she was started on cefepime renal dose of 2 g every 12h. Plasma concentration of cefepime was not measured because the analytical technique was not available in our hospital. After 5th day of cefepime use, the patient became stupor and developed one episode of brief generalized myoclonic seizure. The neurologic examination revealed change in mental status, and minimal response to noxious pain stimulation. There was no focal neurological sign, pathologic reflex, and neck stiffness. Temperature, vital signs and the rest of the physical examination were normal. The brain magnetic resonance imaging (MRI) including diffusion and gradient echo images revealed no acute lesions which can explain sudden mental status change. Serum blood cell counts, glucose, ammonia and electrolytes were within the normal range. Renal profile showed blood urea nitrogen (BUN) of 27 mg/dL (normal range: 7–25 mg/dL), creatinine of 1.33 mg/dL, creatinine clearance (Ccr) of 25.35 mL/min/1.73m2. The electroencephalograph (EEG) revealed continuous 2–3 Hz generalized sharp and wave (Fig. 1). NCSE was diagnosed and administered lorazepam 4 mg intravenous injection with continuous EEG monitoring were started. She was than further treated with levetiracetam 500 mg and valproic acid 900 mg intravenously. She stay on maintenance doses of valproic acid (900 mg/day) and phenytoin (300 mg/day), clonazepam (1.5 mg/day). Repeated EEG revealed improved after anti-epileptic treatment (Fig. 2). As we discontinued cefepime, considering as a causative agent for NCSE, clinical symptoms improved 3 days after discontinuation of cefepime. She was than diagnosed with cefepime induced non convulsive status epilepticus. Anti-epileptic treatment was than discontinued uneventfully. No relapse occurred.
Discussion
Alteration of mental status in patients with post-operative complications and medical illnesses may have potential causes of toxic metabolic alterations, infections, hypoxia, cerebral stroke or NCSE, etc. EEG is helpful in differentiating different causes. Our patient was diagnosed with NCSE with continuous 2–3 Hz generalized sharp and wave. Cefepime as a causative agent was derived by similar EEG finding of cefepime induced NCSE of previous reports.4,5 And temporal relationship of consciousness change, with onset of clinical symptom in 5 days after the administration of cefepime, and improvement in 3 days after the withdrawal. The typical time period for encephalopathy induced by cephalosporin use is a latency of 1 to 10 days following start of medication, and resolution in 2 to 7 days following discontinuation.6 The clinical symptoms other than consciousness changes have been reported including myoclonic seizure, abnormal behavior, mutism, ataxia, asterixia, hallucinations, tremor, clonus, and hyperreflexia. The brief myoclonic seizure preceding NCSE, as in our patient, has been seen in 9 of 25 case reports.4
Cefepime is a fourth-generation cephalosporin which is bactericidal for a broad spectrum of organisms, including Pseudomonas aeruginosa.7 Cefepime is predominantly cleared by renal excretion and the half-life of cefepime in adults with normal renal function is approximately 2 hours.8 The neurotoxic effects of cephalosporins can range from slurred speech, tremor, confusion, aphasia, agitation, coma, myoclonus, seizure, to NCSE.5 The NCSE, in particular has been reported in patients with renal impairment and with use of third and fourth generation agents such as ceftazidime, ceftriaxone, and cefepime.9,10 The mechanism of cefepime-induced NCSE appears to be r-aminobutyric acid (GABA)-A receptor antagonism, reducing the GABA mediated inhibitory response, therefore generating a pro-epileptogenic activity.11
Since the first description in 1945, several reports of penicillin-induced neurotoxicity involving adults have been reported. Mostly those with acute or chronic renal failure. Pathogenesis of neurotoxicity in patients with renal impairment appears to be mediated by rise in serum concentrations, increased permeability of the blood-barrier as well as buildup of toxic organic acids within the cerebrospinal fluid.12 Increased circulating unbound antibiotic also contributes to the vulnerability of patients to CNS toxicity, especially in renal impairment.13 With much emphasis on renal function to neurotoxic effects of cefepime, NCSE have been reported mostly in patients with acute or chronic renal failure with only one case report with normal renal failure.4 However, even in this case of normal renal function, subtle renal impairment was seen with below normal range of creatinine clearance of 44.47 mL/kg/min.14
Our case is an example of NCSE due to neurotoxicity of cephalosporin at therapeutic dose. And it is different from previous cases in the aspect that renal function was recovered in our patient and her creatinine level was within normal limit. In previous cases, cessation of cefepime with hemodialysis treatment helped patient to recover mental status. However, our patient recover consciousness only with stopping cefepime. Thus, our case suggest more strongly that cefepime induced neurotoxicity may occur in patients with renal dose treatment and with recent history of acute renal failure.
In conclusion, awareness of the potential neurotoxic clinical manifestations of various antibiotics and high degree of vigilance in critically ill patients is essential in identifying potentially serious though reversible complications of antibiotic therapy particularly with the advent of newer antimicrobial agents.