Recurrent Seizures Following Focal Motor Status Epilepticus in a Patient with Non-Ketotic Hyperglycemia and Acute Cerebral Infarction
Article information
Abstract
Focal motor status epilepticus (FMSE) is often associated non-ketotic hyperglycemia (NKH). There are no previous reports describing FMSE with NKH that was accompanied by an acute cerebral infarction and its long term follow-up result. We describe the case of a patient having focal motor status epilepticus (FMSE) associated with non-ketotic hyperglycemia (NKH) and acute cerebral infarction who later developed recurrent unprovoked seizures. A small acute infarct was observed in the left frontal subcortical area on diffusion-weighted images (DWI). FMSE was initially controlled with short term antiepileptic drugs and strict glucose control. Two years later, recurrent seizures occurred, and long-term antiepileptic drug treatment was administered. DWI should be considered for acute cerebral infarction in patients having FMSE associated with NKH, and careful follow-up should be conducted for such patients.
Introduction
Focal motor status epilepticus (FMSE) is characterized by repetitive and persistent myoclonic, clonic, or tonic contractions of the arm, face, or neck that can affect an entire side of the body, with or without alteration of mental functions. FMSE can be associated with several medical conditions including brain tumors, metabolic disturbances such as hyponatremia or a hyperosmolar state, and focal cerebral lesions resulting from stroke.1,2
FMSE is often related to non-ketotic hyperglycemia (NKH) and accompanied by an underlying localized brain lesion.3,4 However, we are unaware of any report describing FMSE with NKH that was accompanied by an acute cerebral infarction. Furthermore, little is known as to whether it is epileptogenic or not. Here, we report the case of a patient who presented with FMSE associated with NKH and a magnetic resonance image (MRI)-documented acute cerebral infarction, and later developed recurrent seizures.
Case
A 46-year-old woman presented with sudden and progressive erratic movements of the right hand and arm that had developed 3 days before visiting our hospital. On inspection, initial myoclonus of the right hand turned into a tonic contraction that spread to the wrist and forearm and persisted for approximately 30 seconds. The frequency of seizures gradually increased from once an hour at initial symptom onset, to once every 5 minutes 3 days later. She reported feeling tingling sensations in her right hand and arm prior to seizure onset. She was having diabetes mellitus for more than 15 years, but reported no previous stroke or seizure. The patient was alert and oriented. No focal neurological abnormality, other than partial seizures, was observed. Serum glucose level, osmolality, and sodium level were 690 mg/dL, 312 mOsm/L, and 128 mEq/L, respectively. Test for ketone bodies was negative.
Electroencephalogram (EEG) was normal during the ictal phase. MRI demonstrated a small focal acute infarction in the left frontal subcortical area on a diffusion-weighted image (DWI). A focal cortical hyperperfusion in the left central area was observed on 99m-Tc ECD single photon emission computed tomography (SPECT), which disappeared 2 weeks later. Magnetic resonance angiogram (MRA) and transfemoral cerebral angiogram (TFCA) revealed atherosclerotic stenosis of the left internal carotid artery (ICA) (Fig. 1).
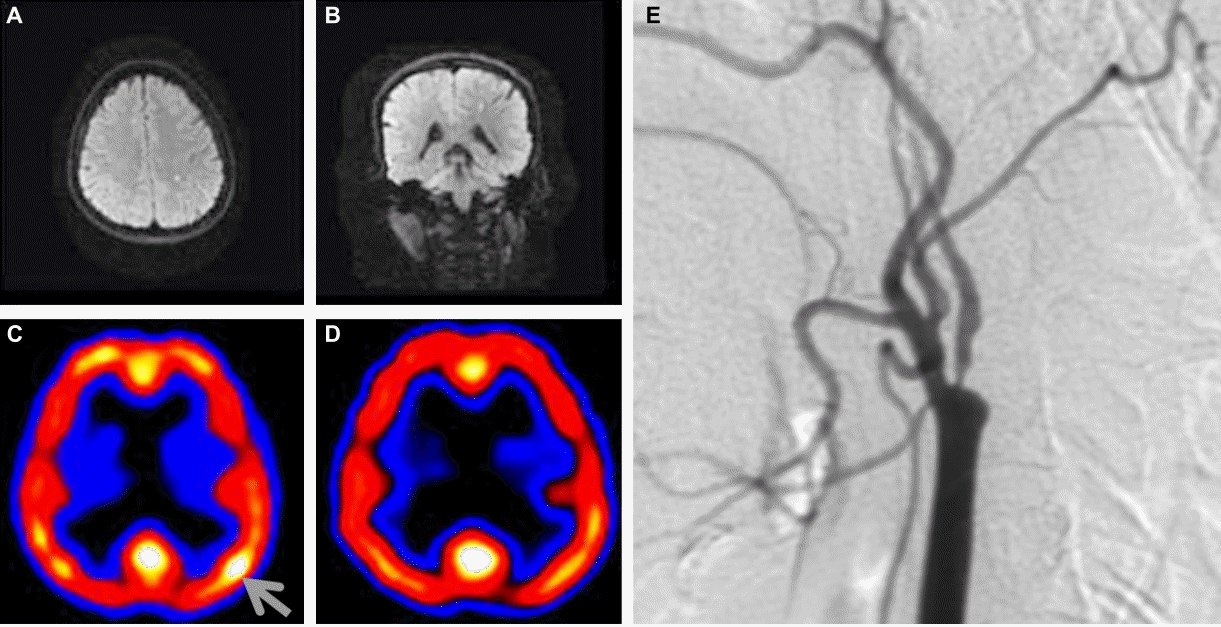
Axial (A) and coronal (B) views of diffusion-weighted images show a small focal infarct in the left frontal subcortical area. 99m-Tc ECD SPECT shows a focal hyperperfusion (grey arrow) in the left central area (C), which disappeared 2 weeks later (D). Severe atherosclerotic stenosis is indicated in the transfemoral cerebral angiography (E).
We administered antiepileptic drugs (carbamazepine CR [600 mg/d after loading with 20 mg/Kg] and valproate [1,000 mg/d]) for 2 weeks and maintained her glucose levels below 250 mg/dL with insulin. The clinical seizures were not observed after 2 days, and she was discharged without sequel. After discharge, glucose control was performed at the Department of Endocrinology.
Two years later, she visited the Department of Neurology because of recurrent generalized tonic-clonic seizures. During the previous month, she had experienced 2 seizures while sleeping. Her fasting and 2-hour postprandial glucose levels were 125 mg/dL and 198 mg/dL, respectively. HbA1c level was 7.5% (2.7–5.8%). Routine chemistry and assays for electrolytes and serum osmolality did not indicate any abnormality, except for mild elevation of serum creatinine level (1.4 mg/dL). A follow-up MRI failed to indicate recurrent strokes or lesions other than a previous small infarction, and interictal EEG was normal. She has been seizure-free for 3 years with carbamazepine treatment.
Discussion
Seizures associated with NKH are well documented. Such seizures can be a presenting symptom of hyperglycemia without ketoacidosis.5 Increased metabolism of gamma-aminobutyric acid triggered by hyperglycemia may lower seizure threshold.6 Associated metabolic disturbances, such as mild hyperosmolality and mild hyponatremia, may contribute to the seizures.3,7 Ketosis is known to have an anticonvulsant effect, and the direct stabilizing effect of ketone bodies and accompanying acidosis may play an important role in preventing seizures.8 Therefore, seizures are more frequently developed in patients with NKH than in patients with diabetic ketoacidosis. Even though the underlying mechanisms are not fully understood, seizures associated with NKH are not rare in clinical practice.
Focal manifestation is difficult to explain. Clinical symptoms typically originate from a focal cerebral lesion, whereas hyperglycemia affects the entire brain. These symptoms may result from previous underlying structural lesions that are more susceptible to the proconvulsant effects of NKH. A previous study reported that the majority of patients examined showed evidence of localized structural cerebral lesions.3,4 In contrast, pre-existing or acute structural lesions were not found in other case reports.7,9–12 Our patient had a small focal subcortical infarct, as revealed by DWI, the location of which was relevant to the patient’s symptoms. In previous reports, DWI was not used for evaluation, and small acute infarctions could have been missed on CT or routine MRI. DWI is essential to reveal accompanying cerebral infarctions in patients with FMSE.
Limb shaking resulting from transient ischemic attacks (TIAs) should be differentiated from FMSE in patients who have carotid artery disease. In our case, this differentiation can be made with several points. Firstly, limb-shaking TIAs usually do not present with Jacksonian march as the attack experienced by our patient did. Secondly, TIA symptoms persist less than 5 minutes and are often accompanied by hemiparesis, which was inconsistent with our patient’s symptoms.13,14 Moreover, brain SPECT showed hyperperfusion in the area relevant to the patient's seizures. Based on the above findings, the abnormal arm movements observed in our patient appear unrelated to TIA.
EEG is a useful diagnostic tool for detection and classification of seizures. However, conventional ictal scalp EEG often fails to detect seizure activities in FMSE because of a limited discharges originated from central sulcus.2,15 In these cases, myoclonus-locked EEG back-averaging technique may be helpful in demonstrating the correlation of the myoclonus with paroxysmal discharges in the motor cortex.16 However, typical clinical manifestations with relevant SPECT finding of our patient are sufficient for the diagnosis.
Patients with histories of status epilepticus have a higher risk of recurrent unprovoked seizures, and the risk is much higher in patients with acute structural brain lesions than in patients with metabolic disturnbances.17,18 We usually administer antiepileptic drugs during the acute period of status epilepticus. However, long-term pro-phylactic antiepileptic treatment is not generally recommended because evidence for effectiveness of such long-term treatment in preventing epileptogenesis is still lacking.16,17–19
In conclusion, our patient experienced acute symptomatic FMSE and subsequent recurrent unprovoked seizures, that is, epilepsy. On the basis of our experience, we suggest that patients with NKH and FMSE may have small cerebral infarctions, and DWI is crucial for detecting such lesions. Furthermore, clinicians should pay careful attention to the emergence of epilepsy during follow-up.