A Muliticenter Retrospective Study of the Actual Using Patterns and Clinical Effects of Topiramate in Patients with Neurosurgical Disease
Article information
Abstract
Background and Purpose:
This study is to investigate the actual using patterns and clinical effects of topiramate in patients with neurosurgical disease as antiepileptic drugs (AEDs) in 94 korean multicenters.
Methods:
A total of 7,152 patients who had taken topiramate for at least 3 months between August 2008 and February 2009 were eligible to participate in this study. We evaluated demographic data, disease entities, duration of topiramate administration, initial and subsequent dosage adjustment, concomitant AEDs, the frequency of seizure reduction, and adverse events.
Results:
Topiramate was commonly prescribed in stroke (38%) and head trauma group (36%). In the dosage of topiramate, the mean initial dosage was 65 mg/day, and the mean maintenance dosage was adjusted into 105 mg/day. The mean duration of the initial dosage for topiramate administration was 24 days, and the mean duration of the maintenance dosage was 125 days, respectively. Among groups with prophylactic administration, 98% did not develop convulsion and among groups with therapeutic administration, 2% was ineffective to control seizure. After taking topiramate, 2% patients showed adverse events, that sensory aberration was the most common.
Conclusions:
These results suggest that topiramate prescribe widely in diverse neurosurgical disorders, and effective in reduction of seizure frequency, and does not cause serious adverse effects comparable with old AEDs.
Introduction
Despite the difference in prevalence rate according to types of diseases such as brain tumor, cerebrovascular diseases and brain damage, epilepsy is one of the diseases commonly occurring in the neurosurgical field. Patients with brain tumor show approximately 4% of epilepsy incidence rate,1 but the rate can be up to 30% depending on the type of tumors.2 In case of subarachnoid hemorrhage, a recent survey reported an incidence rate of 5–8%.3 Another long-term prognosis survey of 1,000 patients undergoing supratentorial surgery reported an epilepsy incidence rate of 17%.4 Therefore, an anticonvulsant is one of the drugs most commonly prescribed by neurosurgeons, and the most commonly used anticonvulsants include valproate, phenytoin, and carbamazepine.5
However, there have been constant concerns about the adverse events of those drugs including hepatotoxicity, weight gain and bone density decreased, resulting in increased use of topiramate as an alternative. Topiramate shows excellent reduction in seizure rate and can control adverse events properly.6 The drug also has additional benefits like various mechanisms of action,7 protection of nerve cells,8,9 and no need for blood concentration monitoring.10 However, there is a big difference of opinion about the reasonable dose of topiramate even among neurosurgeons. Dosage of topiramate can be various depending on the type of a disease in a patient and the degree of its surgical operation. In 2004, American Association of Neurological Surgeons (AANS) conducted the survey of its members to examine their anticonvulsant use and reported a considerable variation in anticonvulsant use according to various factors.5
In Korea, there are no nationwide surveys of anticonvulsant use including topiramate. Thus, we conduct the nationwide retrospective survey to examine the practical using status of topiramate in the neurosurgical field.
Methods
Patients
We retrospectively identified a total of 7,152 patients who have taken topiramate for at least 3 months during the period from August 2008 to February 2009 by the electronic data capture (eDC) system. Ninety-four major hospitals in Korea were involved in this study. The patients were eligible if patients with neurosurgical disease who have taken topiramate for at least 3 months during the period from August 2008 to February 2009. We reviewed each patient’s medical and surgical history, duration of topiramate treatment, initial and maintenance dose, and concomitant drugs and to examine reduction of seizure rate and adverse event incidence according to the topiramate treatment in the Korean neurosurgical field.
Evaluation and assessments
Epilepsy syndrome and seizure classification (Comission on Classification and Terminology of the International League Against Epilepsy, 1989) were based on thorough clinical assessments. Baseline seizure frequency was defined as monthly seizure frequency (i.e., seizure number per month) during the 3-months baseline period. Mean and median reduction rates of seizure frequency were calculated. Responders were defined as at least 50% seizure frequency reduction compared to baseline.
Mean dosage and duration of topiramate were examined through the subgroup analysis according to various criteria as follows: (1) types of the disease and whether the patient underwent surgery, (2) whether the patient have seizure and types of seizure, (3) whether the patient developed adverse events. Relationship between topiramate and adverse effect are as follows; (1) not related, (2) doubtful, (3) possible, (4) probable, (5) very likely.
Statistical analysis
For statistical analysis, we used a commercially available program, SPSS version 15.0 for Windows (SPSS Inc, Chicago, IL, USA). Reduction of the seizure frequency from baseline was analyzed using a univariate analysis (ANOVA). Student’s t-test for independent samples was used to compare continuous clinical and demographic variables. Differences between subgroups were assessed by means of the Mann-Whitney U-test for independent samples or Fisher’s exact test depending on the scale level. Frequency counts and summary statistics were described as mean±SD. The results from the tests were considered statistically significant at p<0.05.
Results
Demographic data and clinical characteristics
A total of 7,152 patients (4,303 males, 2,849 females) were included in this study. The patients age was 53.0±16.56 years. The common causes of topiramate medication were stroke in 38% (n=2,748/7,152) and head trauma in 36% (n=2,610/7,152), respectively. The demographic characteristics and underlying etiologies of the epilepsy are summarized in Table 1. Regarding to the medication of anticonvulsant medication before topiramate prescription, 2,739 (38.3%) patients had a past history of medication, and 3,833 (53.6%) patients did not have any history of anticonvulsant medication, and 580 (8.1%) patients were unknown. The number of anticonvulsants administered to patients before topiramate medication was 1 in 2,537 (92.6%), 2 in 179 (6.5%), and more than 3 in 23 (0.9%) (Table 2). As the results of evaluation for the reason of topiramate prescription, 6,551 (91.6%) patients were prescribed by its superior anticonvulsive effect, and 2,431 (34%) and 2,110 (29.5%) patients were chosen due to the unnecessity of blood monitoring and an expectation of neural protective function, respectively. On the other hands, 1,087 patients (15.2%) selected topiramate, which was effective in the remission of migraine (Table 3).
Topiramate dosage and duration of administration
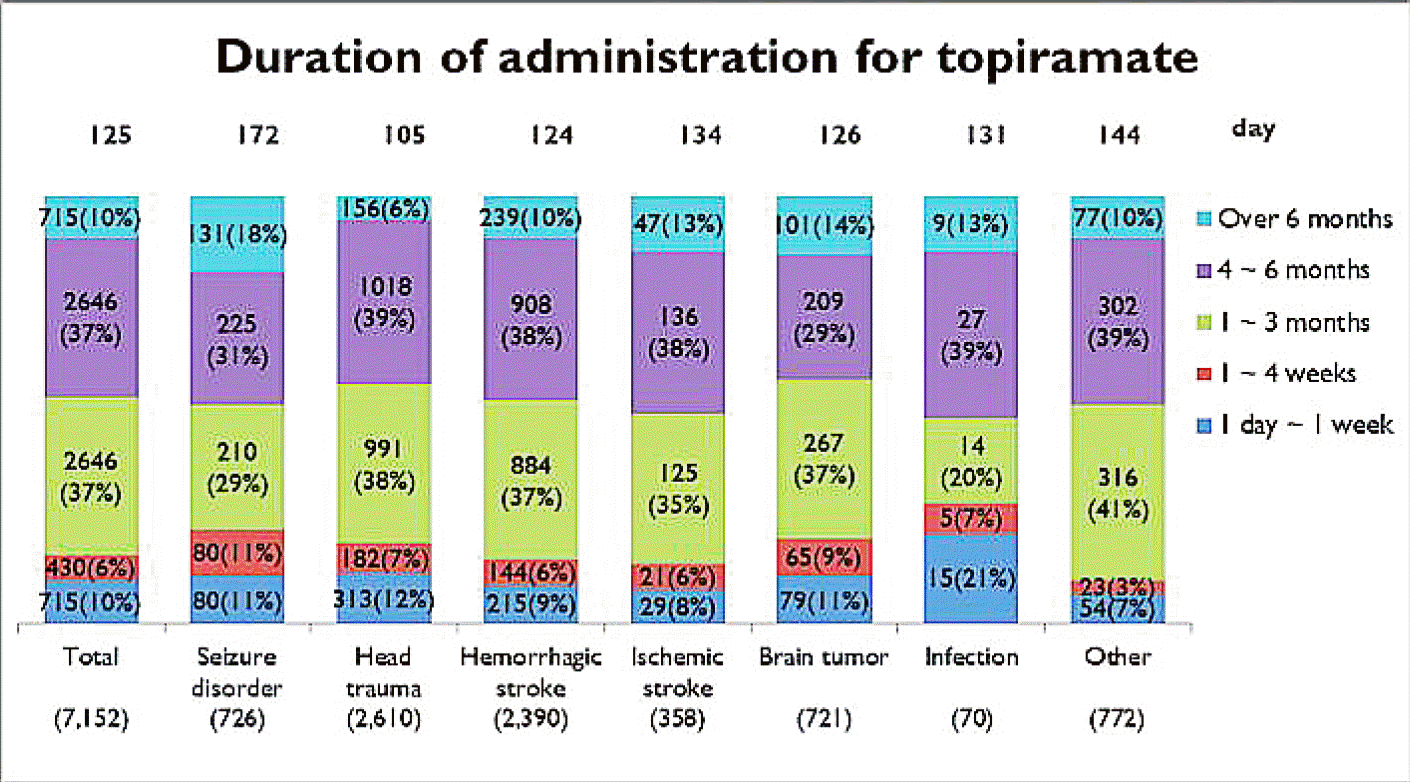
Mean initial dose of topiramate was 65 mg/day, and mean maintenance dosage was increased into 105 mg/day. According to the initial dosage of topiramate based on the type of disease, epilepsy had the highest dose, 82 mg, while the ischemic cerebral disease had the lowest amount, 56 mg. In the respects of maintenance dosage of topiramate based on the type of disease, central nervous system (CNS) infection had the highest dose, 126mg, while the brain tumor had the lowest dose, 97 mg (Fig. 1). The mean duration of initial administration for topiramate was 24 days, and mean duration of maintenance was 125 days. As for administered period depending on the disease types, epilepsy had the longest administered period of 125 days in average, while head injury had the shortest administered period of 105 days (Fig. 2).
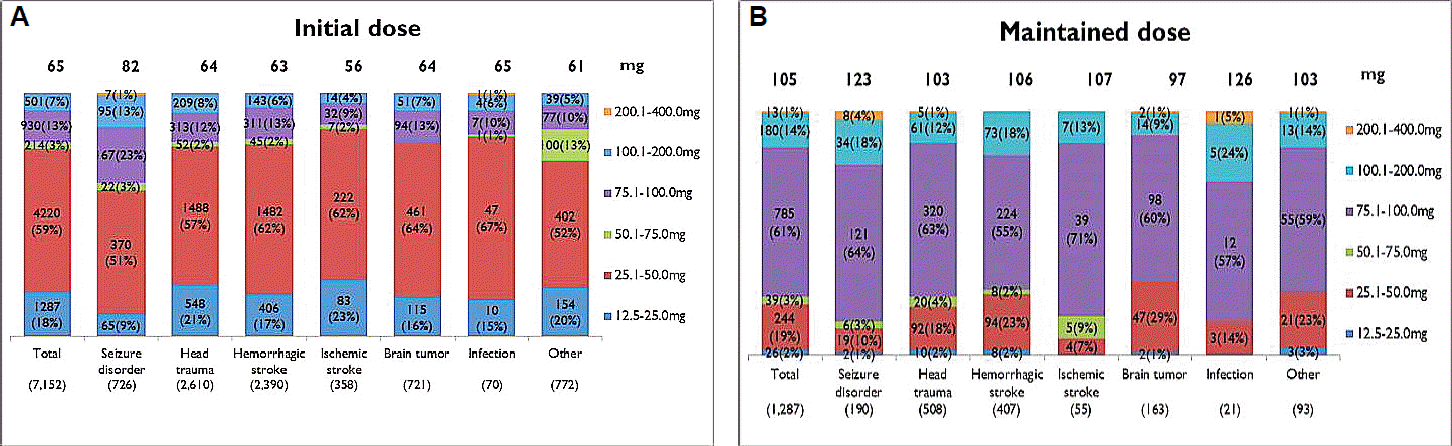
Dosage of topiramate based on the type of disease. Boxes show mean iniatial (A) and maintenance (B) dose according to types of disease entities.
Therapeutic outcome, control of further seizures, and combined regimens
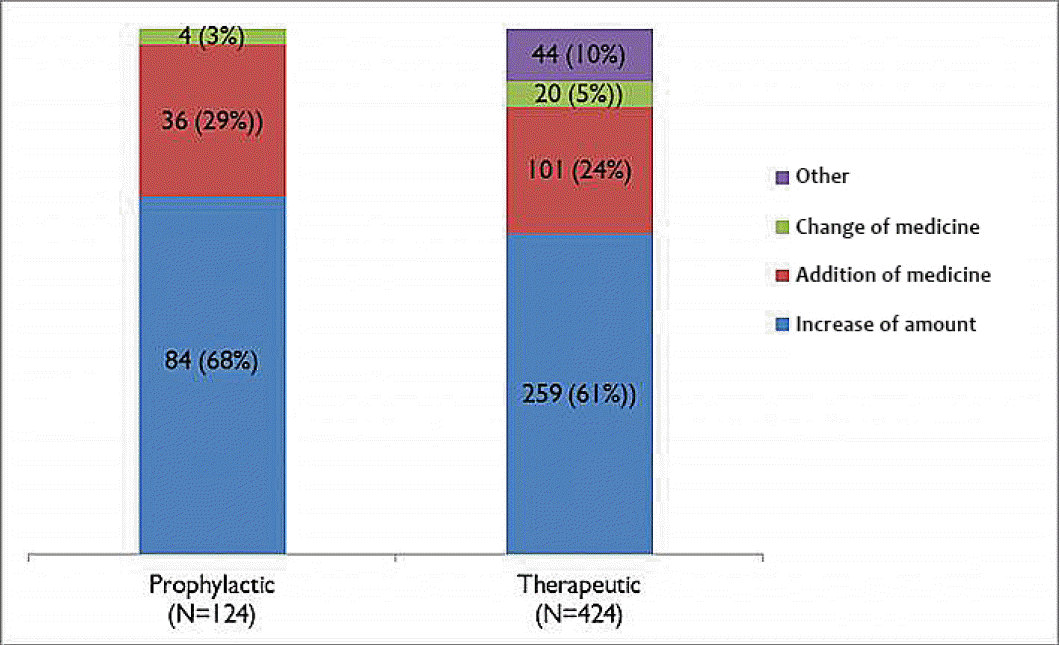
The purpose of topiramate medication was prophylactic for 6,104 (85%) and therapeutic for 1,048 (15%). Among groups with prophylactic purpose, 6,024 (98%) patients did not develop convulsive seizures, but 124 (2%) suffered from seizures. As for the 124 patients with seizure, 50 (40%) patients progressed into the generalized seizures, and 74 (60%) patients had partial seizures. Among groups with therapeutic medication, 637 (60%) patients didn’t develop seizures, 405 (38%) patients experienced reduction in the seizure frequencies, and 20 (2%) patients were ineffective in the treatment of seizure. Among 404 patients with reduced frequency of seizures or ineffectiveness, 273 (64%) patients suffered from generalized seizures, and 151 (36%) patients experienced partial seizures. We applied several therapeutic modalities to manage seizures developed during topiramate medication. Sixty-eight percent (n=88/124) patients were controlled by the dose escalation among groups with prophylactic purpose, and 29% (n=36/124) patients were managed by additional medications. However, among groups (424 patients) with therapeutic purpose, 61% (n=259/424) patients were controlled by dose escalation, while 24% (n=101/424) patients were treated by additional medications (Fig. 3). On the other hands, the major purpose of combined regimen for topiramate and other anticonvulsants were related to enhance antiepileptic effects in 78% (n=573/731) patients. This tendency was particularly evident in CNS infection (92%), seizure disorder (91%), and brain tumors (88%). Another objectives to combinational medication were to decrease the amount of previously prescribed medicine in 15%, and 5% for non-recovery for oral function and fast, respectively (Fig. 4).
Adverse effects and control of adverse events
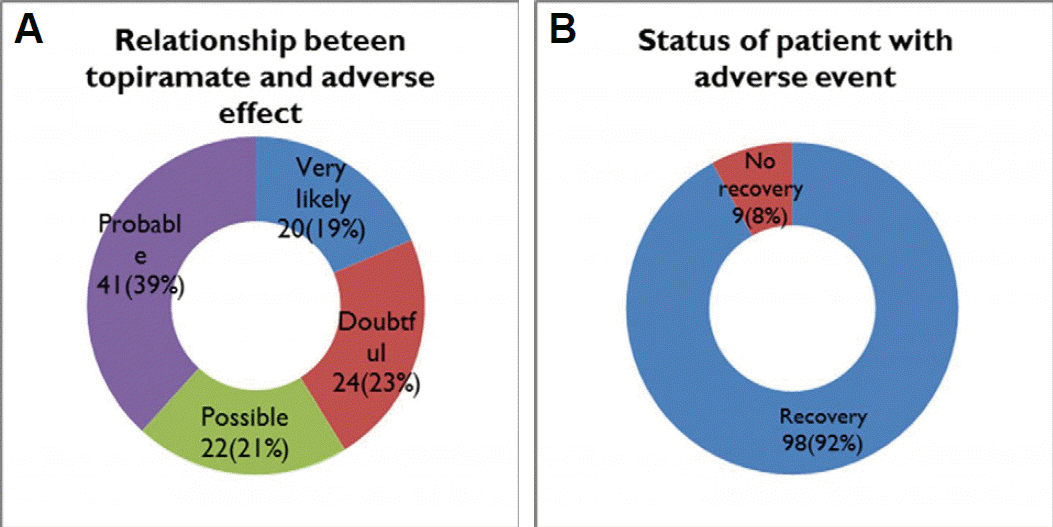
Adverse events of topiramate administration was developed in 102 (2%) patients, and its frequently occurred in patients with the high-dose (≥100 mg) medication, titrated group, and combined regimen (Fig. 5). Sensory aberration was main event in 27 (25.2%) patients. Weight loss, dizziness, and anorexia were also common (Table 4). To manage the adverse effect of topiramate, we closely observed in 62% (n=66/107) patients. However, 13%, 10%, and 11% patients have taken medicine substitution, suspension of prescription, and permanent interruption, respectively. Duration of adverse events in patients mostly stopped within a month, however, 36% (n=39/107) patients suffered from side effects over a month (Fig. 6).
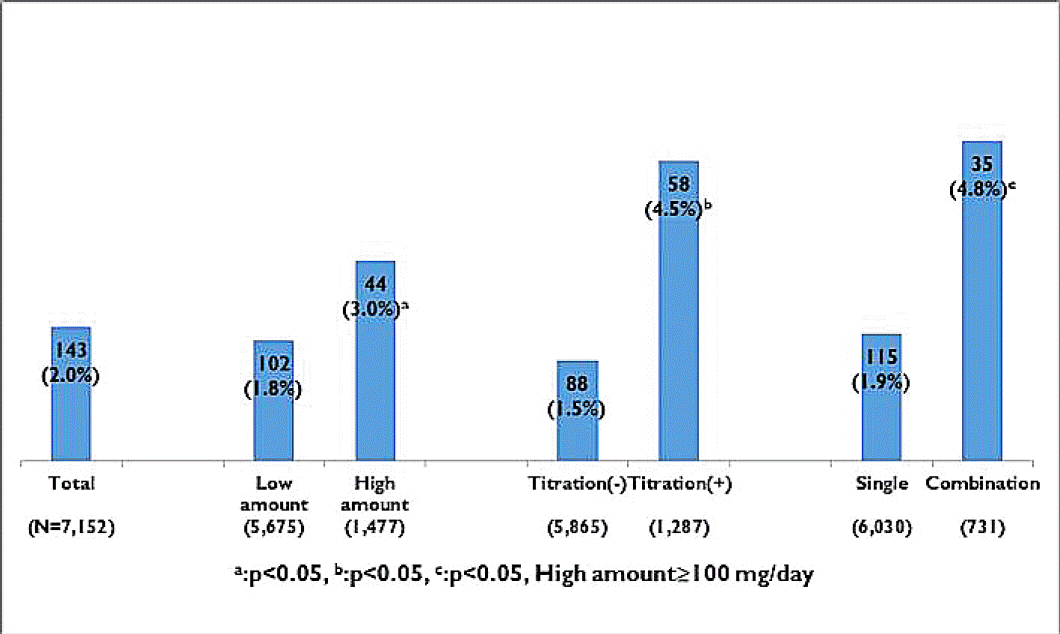
Occurrence rate of adverse events depending on the prescription form. The rate of adverse events of topiramate is higher in patients with high amounts, titrated group and combined regimen (a: p<0.05, b: p<0.05, c: p<0.05, High amount ≥ 100 mg/day)
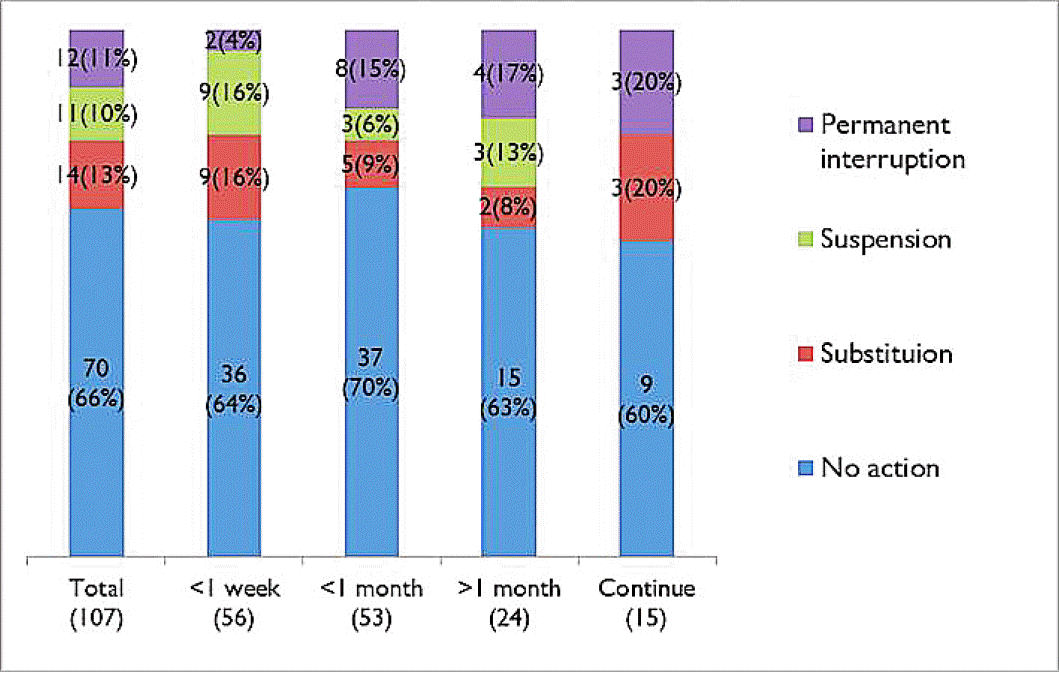
Measures of topiramate depending on the side effect duration. In 64% of patients, adverse events mostly stopped within a month.
A total of 107 abnormal responses for topiramate prescription consisted of very likely in 19%, probable in 39%, possible in 21% and doubtful in 23%, in 8% of whom were patients without recovery from the abnormal responses as follows (Fig. 7).
Discussion
The present study surveyed anamnesis, duration of topiramate administration, initial and maintenance doses of topiramate, and combined drugs in patients with neurosurgical diseases of Korea. In addition, we investigated the reduction ratio of convulsive seizure as a result of taking topiramate and the occurrence of adverse responses.
Topiramate is a derivative of the monosaccharide, D-fructose in the pyranose configuration. Multiple putative mechanisms of action include voltage-sensitive sodium channel blockade,11 calcium channel inhibition,12 increase of potassium conductance,13 GABAA (Gamma-aminobutyric acid) -mediated chloride current increment,14 glutamate-mediated neurotransmission inhibition15 and carbonic an-hydrase isoenzyme inhibition.16
Antiepileptic drugs were used in 65.1% of patients in 3,552 patients with subarachnoid hemorrhage (SAH) in 162 centers and 21 countries between 1991 and 1997.17 In 1973, 60% of clinicians in the USA treated traumatic brain injury (TBI) patients prophylactically with anticonvulsants,18 and a recent survey of clinicians in 127 neurosurgical departments showed 12% prescribe prophylactic anticonvulsants for all head injury patients.19 In the present study, topiramate was most commonly used with stroke and head injury patients groups, with a greater purpose of prevention than that of treatment. In a study of 692 patients, Guerrini reported 42.9% of all patients enrolled had received monotherapy with one antiepileptic drug other than topiramate. The main prior anticonvulsants were carbamazepine, valproate, phenytoin, oxcarbazepine and lamotrigine, respectively. And the reason for switching to topiramate was failure of efficacy in 61.6% of patients, failure of tolerability in 24.9% and both in 13.1%.20 According to a research published in Taiwan from 2003 to 2007, the most commonly used first-generation anticonvulsants was phenytoin and second-generation anticonvulsants were topiramate, levetiracetam, oxcarbazepine followed.21 On the other hand, valproate was most used among anticonvulsants in this study, since the reason for prescribing topiramate was its excellent anticonvulsant effects.
The manufacturer recommends a starting dose of 50 mg/day for adults, but it is often a better strategy to begin with 25 mg/day with increases no faster than 25 mg/day week, unless there is a need for haste.22 In an open-label trial involving 441 adult patients, primarily in Europe, the average monotherapy dose achieved was 125 mg/day (range 25–700 mg/day).20 Another large naturalistic trail in the United States assessed adjunctive therapy in adults, mostly with enzyme inducing drugs: final doses were most commonly in the 300–350 mg/day range.23 In our study, mean initial dose was 65 mg/day and mean maintenance dose was 105 mg/day, where the initial dose was highest for epilepsy, the maintenance dose being maintained highest for CNS infectious diseases.
Biton et al. investigated in a double-blind placebo controlled study of 80 patients aged 3 to 59 years. The median percentage seizure reduction rate was 56.7% for topiramate patients and 9% for placebo patients (p=0.019). The proportion of patients with 50% or higher reduction in primary generalized tonic-clonic (PGTC) seizure rate was 22/39 (56%) and 8/40 (20%) for the topiramate and placebo groups, respectively (p=0.001).24 In other study of 671 patients, Guerrini reported 44.3% of patients were seizure-free and 72.0–83.0% of patient achieved at least 50% seizure reduction. In the group with focal epilepsies, 39.4% of patients were seizure-free, while 73.9% achieved at least 50% seizure control. In the group with generalized epilepsies, 61.5% remained in remission, while 83.8% achieved at least 50% seizure reduction.20 Arroyo et al.25 reported that the second trial used an identical design except that patients 6 years and older were enrolled and the low-dose target was 50 mg/day, the high-dose target 400 mg/day. Time to the first seizure favored the high dose, with seizure-free rates of 59% and 76% for the low and high doses respectively (p=0.001). 637 (60%) of 1,048 patients with whom topiramate was used for therapeutic purpose in the present study were seizure-free, and 405 (38%) showed a decrease in convulsion. 6,024 (98%) of 6,104 patients for whom the drug was administered for prophylactic purpose were seizure-free, which showed a higher effect than in other studies.
Topiramate is a relatively safe AED, considering its lack of significant effects on cardiovascular function, bone density, bone marrow cells and thyroid function.26 The central nervous system represented the most common side effect that consisted of somnolence, fatigue, headache, psychomotor slowing, confusion, difficulty with memory, impaired concentration and attention, speech and language problems.27 The other side effects are renal calculi, ophthalmologic side effects, metabolic acidosis, weight loss, hypohydrosis, heat intolerance and hyperthermia. Renal calculi have been observed in 1.5% of treated patients.28 Ophthalmological side effects are acute glaucoma, acute myopia, suprachoroidal effusion, periorbital edema and scleritis.29 Hyperchloraemic, non-anion gap metabolic acidosis has been observed that up to 70% of children develop >10% decrease in serum bicarbonate on topiramate treatment.30 In the present study, adverse responses occurred with 107 (2%) of the entire patients, of whom abnormal sensation and weight reduction were most common, and 92% of them were recovered. Also it could be seen that adverse responses showed a high occurrence among patient groups with dose escalation, and the combinational regimen with other anticonvulsants.
Acknowledgements
This study was supported by Janssen Korea Pharmaceutical Ltd.